From Wikipedia, the free encyclopedia
https://en.wikipedia.org/wiki/Metalloid
A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature of chemistry.
The six commonly recognised metalloids are boron, silicon, germanium, arsenic, antimony and tellurium. Five elements are less frequently so classified: carbon, aluminium, selenium, polonium and astatine. On a standard periodic table, all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right. Some periodic tables include a dividing line between metals and nonmetals, and the metalloids may be found close to this line.
Typical metalloids have a metallic appearance, but they are brittle and only fair conductors of electricity. Chemically, they behave mostly as nonmetals. They can form alloys with metals. Most of their other physical properties and chemical properties are intermediate in nature. Metalloids are usually too brittle to have any structural uses. They and their compounds are used in alloys, biological agents, catalysts, flame retardants, glasses, optical storage and optoelectronics, pyrotechnics, semiconductors, and electronics.
The electrical properties of silicon and germanium enabled the establishment of the semiconductor industry in the 1950s and the development of solid-state electronics from the early 1960s.
The term metalloid originally referred to nonmetals. Its more recent meaning, as a category of elements with intermediate or hybrid properties, became widespread in 1940–1960. Metalloids are sometimes called semimetals, a practice that has been discouraged, as the term semimetal has a different meaning in physics than in chemistry. In physics, it refers to a specific kind of electronic band structure of a substance. In this context, only arsenic and antimony are semimetals, and commonly recognised as metalloids.
Definitions
Judgment-based
A metalloid is an element that possesses a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals, and which is therefore hard to classify as either a metal or a nonmetal. This is a generic definition that draws on metalloid attributes consistently cited in the literature. Difficulty of categorisation is a key attribute. Most elements have a mixture of metallic and nonmetallic properties, and can be classified according to which set of properties is more pronounced. Only the elements at or near the margins, lacking a sufficiently clear preponderance of either metallic or nonmetallic properties, are classified as metalloids.
Boron, silicon, germanium, arsenic, antimony, and tellurium are commonly recognised as metalloids. Depending on the author, one or more from selenium, polonium, or astatine are sometimes added to the list. Boron sometimes is excluded, by itself, or with silicon. Sometimes tellurium is not regarded as a metalloid. The inclusion of antimony, polonium, and astatine as metalloids has been questioned.
Other elements are occasionally classified as metalloids. These elements include hydrogen, beryllium, nitrogen, phosphorus, sulfur, zinc, gallium, tin, iodine, lead, bismuth, and radon. The term metalloid has also been used for elements that exhibit metallic lustre and electrical conductivity, and that are amphoteric, such as arsenic, antimony, vanadium, chromium, molybdenum, tungsten, tin, lead, and aluminium. The p-block metals, and nonmetals (such as carbon or nitrogen) that can form alloys with metals or modify their properties have also occasionally been considered as metalloids.
Criteria-based
| Element | IE (kcal/mol) |
IE (kJ/mol) |
EN | Band structure |
|---|---|---|---|---|
| Boron | 191 | 801 | 2.04 | semiconductor |
| Silicon | 188 | 787 | 1.90 | semiconductor |
| Germanium | 182 | 762 | 2.01 | semiconductor |
| Arsenic | 226 | 944 | 2.18 | semimetal |
| Antimony | 199 | 831 | 2.05 | semimetal |
| Tellurium | 208 | 869 | 2.10 | semiconductor |
| average | 199 | 832 | 2.05 |
|
| The elements commonly recognised as metalloids, and their ionization energies (IE); electronegativities (EN, revised Pauling scale); and electronic band structures (most thermodynamically-stable forms under ambient conditions). | ||||
No widely accepted definition of a metalloid exists, nor any division of the periodic table into metals, metalloids, and nonmetals; Hawkes questioned the feasibility of establishing a specific definition, noting that anomalies can be found in several attempted constructs. Classifying an element as a metalloid has been described by Sharp as "arbitrary".
The number and identities of metalloids depend on what classification criteria are used. Emsley recognised four metalloids (germanium, arsenic, antimony, and tellurium); James et al. listed twelve (Emsley's plus boron, carbon, silicon, selenium, bismuth, polonium, moscovium, and livermorium). On average, seven elements are included in such lists; individual classification arrangements tend to share common ground and vary in the ill-defined margins.
A single quantitative criterion such as electronegativity is commonly used, metalloids having electronegativity values from 1.8 or 1.9 to 2.2. Further examples include packing efficiency (the fraction of volume in a crystal structure occupied by atoms) and the Goldhammer-Herzfeld criterion ratio. The commonly recognised metalloids have packing efficiencies of between 34% and 41%. The Goldhammer-Herzfeld ratio, roughly equal to the cube of the atomic radius divided by the molar volume, is a simple measure of how metallic an element is, the recognised metalloids having ratios from around 0.85 to 1.1 and averaging 1.0. Other authors have relied on, for example, atomic conductance or bulk coordination number.
Jones, writing on the role of classification in science, observed that "[classes] are usually defined by more than two attributes". Masterton and Slowinski used three criteria to describe the six elements commonly recognised as metalloids: metalloids have ionization energies around 200 kcal/mol (837 kJ/mol) and electronegativity values close to 2.0. They also said that metalloids are typically semiconductors, though antimony and arsenic (semimetals from a physics perspective) have electrical conductivities approaching those of metals. Selenium and polonium are suspected as not in this scheme, while astatine's status is uncertain.
In this context, Vernon proposed that a metalloid is a chemical element that, in its standard state, has (a) the electronic band structure of a semiconductor or a semimetal; and (b) an intermediate first ionization potential "(say 750−1,000 kJ/mol)"; and (c) an intermediate electronegativity (1.9–2.2).
Periodic table territory
|
|
1 | 2 |
|
12 | 13 | 14 | 15 | 16 | 17 | 18 |
|
|
| |||||||||||
|
|
H |
|
|
|
|
|
|
He |
| ||
|
|
Li | Be |
|
|
B | C | N | O | F | Ne |
|
|
|
Na | Mg |
|
|
Al | Si | P | S | Cl | Ar |
|
|
|
K | Ca |
|
Zn | Ga | Ge | As | Se | Br | Kr |
|
|
|
Rb | Sr |
|
Cd | In | Sn | Sb | Te | I | Xe |
|
|
|
Cs | Ba |
|
Hg | Tl | Pb | Bi | Po | At | Rn |
|
|
|
Fr | Ra |
|
Cn | Nh | Fl | Mc | Lv | Ts | Og |
|
|
|
| ||||||||||
|
|
Commonly (93%) to rarely (9%) recognised as a
metalloid: B, C, Al, Si, Ge, As, Se, Sb, Te, Po, At Very rarely (1–5%): H, Be, P, S, Ga, Sn, I, Pb, Bi, Fl, Mc, Lv, Ts
Sporadically: N, Zn, Rn
Metal–nonmetal dividing line: between H and Li, Be and B, Al and Si, Ge and As, Sb and Te, Po and At, and Ts and Og
|
| |||||||||
|
Periodic table extract showing groups 1–2 and 12–18, and a dividing line between metals and nonmetals. Percentages are median appearance frequencies in the list of metalloid lists. Sporadically recognised elements show that the metalloid net is sometimes cast very widely; although they do not appear in the list of metalloid lists, isolated references to their designation as metalloids can be found in the literature (as cited in this article). |
| ||||||||||
Location
Metalloids lie on either side of the dividing line between metals and nonmetals. This can be found, in varying configurations, on some periodic tables. Elements to the lower left of the line generally display increasing metallic behaviour; elements to the upper right display increasing nonmetallic behaviour. When presented as a regular stairstep, elements with the highest critical temperature for their groups (Li, Be, Al, Ge, Sb, Po) lie just below the line.
The diagonal positioning of the metalloids represents an exception to the observation that elements with similar properties tend to occur in vertical groups. A related effect can be seen in other diagonal similarities between some elements and their lower right neighbours, specifically lithium-magnesium, beryllium-aluminium, and boron-silicon. Rayner-Canham has argued that these similarities extend to carbon-phosphorus, nitrogen-sulfur, and into three d-block series.
This exception arises due to competing horizontal and vertical trends in the nuclear charge. Going along a period, the nuclear charge increases with atomic number as do the number of electrons. The additional pull on outer electrons as nuclear charge increases generally outweighs the screening effect of having more electrons. With some irregularities, atoms therefore become smaller, ionization energy increases, and there is a gradual change in character, across a period, from strongly metallic, to weakly metallic, to weakly nonmetallic, to strongly nonmetallic elements. Going down a main group, the effect of increasing nuclear charge is generally outweighed by the effect of additional electrons being further away from the nucleus. Atoms generally become larger, ionization energy falls, and metallic character increases. The net effect is that the location of the metal–nonmetal transition zone shifts to the right in going down a group, and analogous diagonal similarities are seen elsewhere in the periodic table, as noted.
Alternative treatments
Elements bordering the metal–nonmetal dividing line are not always classified as metalloids, noting a binary classification can facilitate the establishment of rules for determining bond types between metals and nonmetals. In such cases, the authors concerned focus on one or more attributes of interest to make their classification decisions, rather than being concerned about the marginal nature of the elements in question. Their considerations may or not be made explicit and may, at times, seem arbitrary. Metalloids may be grouped with metals; or regarded as nonmetals; or treated as a sub-category of nonmetals. Other authors have suggested classifying some elements as metalloids "emphasizes that properties change gradually rather than abruptly as one moves across or down the periodic table". Some periodic tables distinguish elements that are metalloids and display no formal dividing line between metals and nonmetals. Metalloids are instead shown as occurring in a diagonal band or diffuse region. The key consideration is to explain the context for the taxonomy in use.
Properties
Metalloids usually look like metals but behave largely like nonmetals. Physically, they are shiny, brittle solids with intermediate to relatively good electrical conductivity and the electronic band structure of a semimetal or semiconductor. Chemically, they mostly behave as (weak) nonmetals, have intermediate ionization energies and electronegativity values, and amphoteric or weakly acidic oxides. They can form alloys with metals. Most of their other physical and chemical properties are intermediate in nature.
Compared to metals and nonmetals
Characteristic properties of metals, metalloids, and nonmetals are summarized in the table. Physical properties are listed in order of ease of determination; chemical properties run from general to specific, and then to descriptive.
| Physical property | Metals | Metalloids | Nonmetals |
|---|---|---|---|
| Form | solid; a few liquid at or near room temperature (Ga, Hg, Rb, Cs, Fr) | solid | majority gaseous |
| Appearance | lustrous (at least when freshly fractured) | lustrous | several colourless; others coloured, or metallic grey to black |
| Elasticity | typically elastic, ductile, malleable (when solid) | brittle | brittle, if solid |
| Electrical conductivity | good to high | intermediate to good | poor to good |
| Band structure | metallic (Bi = semimetallic) | are semiconductors or, if not (As, Sb = semimetallic), exist in semiconducting forms | semiconductor or insulator |
| Chemical property | Metals | Metalloids | Nonmetals |
| General chemical behaviour | metallic | nonmetallic | nonmetallic |
| Ionization energy | relatively low | intermediate ionization energies, usually falling between those of metals and nonmetals | relatively high |
| Electronegativity | usually low | have electronegativity values close to 2 (revised Pauling scale) or within the range of 1.9–2.2 (Allen scale) | high |
| When mixed with metals |
give alloys | can form alloys | ionic or interstitial compounds formed |
| Oxides | lower oxides basic; higher oxides increasingly acidic | amphoteric or weakly acidic | acidic |
The above table reflects the hybrid nature of metalloids. The properties of form, appearance, and behaviour when mixed with metals are more like metals. Elasticity and general chemical behaviour are more like nonmetals. Electrical conductivity, band structure, ionization energy, electronegativity, and oxides are intermediate between the two.
Common applications
- The focus of this section is on the recognised metalloids. Elements less often recognised as metalloids are ordinarily classified as either metals or nonmetals; some of these are included here for comparative purposes.
Metalloids are too brittle to have any structural uses in their pure forms. They and their compounds are used as (or in) alloying components, biological agents (toxicological, nutritional, and medicinal), catalysts, flame retardants, glasses (oxide and metallic), optical storage media and optoelectronics, pyrotechnics, semiconductors, and electronics.
Alloys

Writing early in the history of intermetallic compounds, the British metallurgist Cecil Desch observed that "certain non-metallic elements are capable of forming compounds of distinctly metallic character with metals, and these elements may therefore enter into the composition of alloys". He associated silicon, arsenic, and tellurium, in particular, with the alloy-forming elements. Phillips and Williams suggested that compounds of silicon, germanium, arsenic, and antimony with B metals, "are probably best classed as alloys".
Among the lighter metalloids, alloys with transition metals are well-represented. Boron can form intermetallic compounds and alloys with such metals of the composition MnB, if n > 2. Ferroboron (15% boron) is used to introduce boron into steel; nickel-boron alloys are ingredients in welding alloys and case hardening compositions for the engineering industry. Alloys of silicon with iron and with aluminium are widely used by the steel and automotive industries, respectively. Germanium forms many alloys, most importantly with the coinage metals.
The heavier metalloids continue the theme. Arsenic can form alloys with metals, including platinum and copper; it is also added to copper and its alloys to improve corrosion resistance and appears to confer the same benefit when added to magnesium. Antimony is well known as an alloy-former, including with the coinage metals. Its alloys include pewter (a tin alloy with up to 20% antimony) and type metal (a lead alloy with up to 25% antimony). Tellurium readily alloys with iron, as ferrotellurium (50–58% tellurium), and with copper, in the form of copper tellurium (40–50% tellurium). Ferrotellurium is used as a stabilizer for carbon in steel casting. Of the non-metallic elements less often recognised as metalloids, selenium – in the form of ferroselenium (50–58% selenium) – is used to improve the machinability of stainless steels.
Biological agents

All six of the elements commonly recognised as metalloids have toxic, dietary or medicinal properties. Arsenic and antimony compounds are especially toxic; boron, silicon, and possibly arsenic, are essential trace elements. Boron, silicon, arsenic, and antimony have medical applications, and germanium and tellurium are thought to have potential.
Boron is used in insecticides and herbicides. It is an essential trace element. As boric acid, it has antiseptic, antifungal, and antiviral properties.
Silicon is present in silatrane, a highly toxic rodenticide. Long-term inhalation of silica dust causes silicosis, a fatal disease of the lungs. Silicon is an essential trace element. Silicone gel can be applied to badly burned patients to reduce scarring.
Salts of germanium are potentially harmful to humans and animals if ingested on a prolonged basis. There is interest in the pharmacological actions of germanium compounds but no licensed medicine as yet.
Arsenic is notoriously poisonous and may also be an essential element in ultratrace amounts. During World War I, both sides used "arsenic-based sneezing and vomiting agents…to force enemy soldiers to remove their gas masks before firing mustard or phosgene at them in a second salvo." It has been used as a pharmaceutical agent since antiquity, including for the treatment of syphilis before the development of antibiotics. Arsenic is also a component of melarsoprol, a medicinal drug used in the treatment of human African trypanosomiasis or sleeping sickness. In 2003, arsenic trioxide (under the trade name Trisenox) was re-introduced for the treatment of acute promyelocytic leukaemia, a cancer of the blood and bone marrow. Arsenic in drinking water, which causes lung and bladder cancer, has been associated with a reduction in breast cancer mortality rates.
Metallic antimony is relatively non-toxic, but most antimony compounds are poisonous. Two antimony compounds, sodium stibogluconate and stibophen, are used as antiparasitical drugs.
Elemental tellurium is not considered particularly toxic; two grams of sodium tellurate, if administered, can be lethal. People exposed to small amounts of airborne tellurium exude a foul and persistent garlic-like odour. Tellurium dioxide has been used to treat seborrhoeic dermatitis; other tellurium compounds were used as antimicrobial agents before the development of antibiotics. In the future, such compounds may need to be substituted for antibiotics that have become ineffective due to bacterial resistance.
Of the elements less often recognised as metalloids, beryllium and lead are noted for their toxicity; lead arsenate has been extensively used as an insecticide. Sulfur is one of the oldest of the fungicides and pesticides. Phosphorus, sulfur, zinc, selenium, and iodine are essential nutrients, and aluminium, tin, and lead may be. Sulfur, gallium, selenium, iodine, and bismuth have medicinal applications. Sulfur is a constituent of sulfonamide drugs, still widely used for conditions such as acne and urinary tract infections. Gallium nitrate is used to treat the side effects of cancer; gallium citrate, a radiopharmaceutical, facilitates imaging of inflamed body areas. Selenium sulfide is used in medicinal shampoos and to treat skin infections such as tinea versicolor. Iodine is used as a disinfectant in various forms. Bismuth is an ingredient in some antibacterials.
Catalysts
Boron trifluoride and trichloride are used as catalysts in organic synthesis and electronics; the tribromide is used in the manufacture of diborane. Non-toxic boron ligands could replace toxic phosphorus ligands in some transition metal catalysts. Silica sulfuric acid (SiO2OSO3H) is used in organic reactions. Germanium dioxide is sometimes used as a catalyst in the production of PET plastic for containers; cheaper antimony compounds, such as the trioxide or triacetate, are more commonly employed for the same purpose despite concerns about antimony contamination of food and drinks. Arsenic trioxide has been used in the production of natural gas, to boost the removal of carbon dioxide, as have selenous acid and tellurous acid. Selenium acts as a catalyst in some microorganisms. Tellurium, its dioxide, and its tetrachloride are strong catalysts for air oxidation of carbon above 500 °C. Graphite oxide can be used as a catalyst in the synthesis of imines and their derivatives. Activated carbon and alumina have been used as catalysts for the removal of sulfur contaminants from natural gas. Titanium doped aluminium has been identified as a substitute for expensive noble metal catalysts used in the production of industrial chemicals.
Flame retardants
Compounds of boron, silicon, arsenic, and antimony have been used as flame retardants. Boron, in the form of borax, has been used as a textile flame retardant since at least the 18th century. Silicon compounds such as silicones, silanes, silsesquioxane, silica, and silicates, some of which were developed as alternatives to more toxic halogenated products, can considerably improve the flame retardancy of plastic materials. Arsenic compounds such as sodium arsenite or sodium arsenate are effective flame retardants for wood but have been less frequently used due to their toxicity. Antimony trioxide is a flame retardant. Aluminium hydroxide has been used as a wood-fibre, rubber, plastic, and textile flame retardant since the 1890s. Apart from aluminium hydroxide, use of phosphorus based flame-retardants – in the form of, for example, organophosphates – now exceeds that of any of the other main retardant types. These employ boron, antimony, or halogenated hydrocarbon compounds.
Glass formation

The oxides B2O3, SiO2, GeO2, As2O3, and Sb2O3 readily form glasses. TeO2 forms a glass but this requires a "heroic quench rate" or the addition of an impurity; otherwise the crystalline form results. These compounds are used in chemical, domestic, and industrial glassware and optics. Boron trioxide is used as a glass fibre additive, and is also a component of borosilicate glass, widely used for laboratory glassware and domestic ovenware for its low thermal expansion. Most ordinary glassware is made from silicon dioxide. Germanium dioxide is used as a glass fibre additive, as well as in infrared optical systems. Arsenic trioxide is used in the glass industry as a decolourizing and fining agent (for the removal of bubbles), as is antimony trioxide. Tellurium dioxide finds application in laser and nonlinear optics.
Amorphous metallic glasses are generally most easily prepared if one of the components is a metalloid or "near metalloid" such as boron, carbon, silicon, phosphorus or germanium. Aside from thin films deposited at very low temperatures, the first known metallic glass was an alloy of composition Au75Si25 reported in 1960. A metallic glass having a strength and toughness not previously seen, of composition Pd82.5P6Si9.5Ge2, was reported in 2011.
Phosphorus, selenium, and lead, which are less often recognised as metalloids, are also used in glasses. Phosphate glass has a substrate of phosphorus pentoxide (P2O5), rather than the silica (SiO2) of conventional silicate glasses. It is used, for example, to make sodium lamps. Selenium compounds can be used both as decolourising agents and to add a red colour to glass. Decorative glassware made of traditional lead glass contains at least 30% lead(II) oxide (PbO); lead glass used for radiation shielding may have up to 65% PbO. Lead-based glasses have also been extensively used in electronic components, enamelling, sealing and glazing materials, and solar cells. Bismuth based oxide glasses have emerged as a less toxic replacement for lead in many of these applications.
Optical storage and optoelectronics
Varying compositions of GeSbTe ("GST alloys") and Ag- and In- doped Sb2Te ("AIST alloys"), being examples of phase-change materials, are widely used in rewritable optical discs and phase-change memory devices. By applying heat, they can be switched between amorphous (glassy) and crystalline states. The change in optical and electrical properties can be used for information storage purposes. Future applications for GeSbTe may include, "ultrafast, entirely solid-state displays with nanometre-scale pixels, semi-transparent 'smart' glasses, 'smart' contact lenses, and artificial retina devices."
Pyrotechnics

The recognised metalloids have either pyrotechnic applications or associated properties. Boron and silicon are commonly encountered; they act somewhat like metal fuels. Boron is used in pyrotechnic initiator compositions (for igniting other hard-to-start compositions), and in delay compositions that burn at a constant rate. Boron carbide has been identified as a possible replacement for more toxic barium or hexachloroethane mixtures in smoke munitions, signal flares, and fireworks. Silicon, like boron, is a component of initiator and delay mixtures. Doped germanium can act as a variable speed thermite fuel. Arsenic trisulfide As2S3 was used in old naval signal lights; in fireworks to make white stars; in yellow smoke screen mixtures; and in initiator compositions. Antimony trisulfide Sb2S3 is found in white-light fireworks and in flash and sound mixtures. Tellurium has been used in delay mixtures and in blasting cap initiator compositions.
Carbon, aluminium, phosphorus, and selenium continue the theme. Carbon, in black powder, is a constituent of fireworks rocket propellants, bursting charges, and effects mixtures, and military delay fuses and igniters. Aluminium is a common pyrotechnic ingredient, and is widely employed for its capacity to generate light and heat, including in thermite mixtures. Phosphorus can be found in smoke and incendiary munitions, paper caps used in toy guns, and party poppers. Selenium has been used in the same way as tellurium.
Semiconductors and electronics

All the elements commonly recognised as metalloids (or their compounds) have been used in the semiconductor or solid-state electronic industries.
Some properties of boron have limited its use as a semiconductor. It has a high melting point, single crystals are relatively hard to obtain, and introducing and retaining controlled impurities is difficult.
Silicon is the leading commercial semiconductor; it forms the basis of modern electronics (including standard solar cells) and information and communication technologies. This was despite the study of semiconductors, early in the 20th century, having been regarded as the "physics of dirt" and not deserving of close attention.
Germanium has largely been replaced by silicon in semiconducting devices, being cheaper, more resilient at higher operating temperatures, and easier to work during the microelectronic fabrication process. Germanium is still a constituent of semiconducting silicon-germanium "alloys" and these have been growing in use, particularly for wireless communication devices; such alloys exploit the higher carrier mobility of germanium. The synthesis of gram-scale quantities of semiconducting germanane was reported in 2013. This consists of one-atom thick sheets of hydrogen-terminated germanium atoms, analogous to graphane. It conducts electrons more than ten times faster than silicon and five times faster than germanium, and is thought to have potential for optoelectronic and sensing applications. The development of a germanium-wire based anode that more than doubles the capacity of lithium-ion batteries was reported in 2014. In the same year, Lee et al. reported that defect-free crystals of graphene large enough to have electronic uses could be grown on, and removed from, a germanium substrate.
Arsenic and antimony are not semiconductors in their standard states. Both form type III-V semiconductors (such as GaAs, AlSb or GaInAsSb) in which the average number of valence electrons per atom is the same as that of Group 14 elements. These compounds are preferred for some special applications. Antimony nanocrystals may enable lithium-ion batteries to be replaced by more powerful sodium ion batteries.
Tellurium, which is a semiconductor in its standard state, is used mainly as a component in type II/VI semiconducting-chalcogenides; these have applications in electro-optics and electronics. Cadmium telluride (CdTe) is used in solar modules for its high conversion efficiency, low manufacturing costs, and large band gap of 1.44 eV, letting it absorb a wide range of wavelengths. Bismuth telluride (Bi2Te3), alloyed with selenium and antimony, is a component of thermoelectric devices used for refrigeration or portable power generation.
Five metalloids – boron, silicon, germanium, arsenic, and antimony – can be found in cell phones (along with at least 39 other metals and nonmetals). Tellurium is expected to find such use. Of the less often recognised metalloids, phosphorus, gallium (in particular) and selenium have semiconductor applications. Phosphorus is used in trace amounts as a dopant for n-type semiconductors. The commercial use of gallium compounds is dominated by semiconductor applications – in integrated circuits, cell phones, laser diodes, light-emitting diodes, photodetectors, and solar cells. Selenium is used in the production of solar cells and in high-energy surge protectors.
Boron, silicon, germanium, antimony, and tellurium, as well as heavier metals and metalloids such as Sm, Hg, Tl, Pb, Bi, and Se, can be found in topological insulators. These are alloys or compounds which, at ultracold temperatures or room temperature (depending on their composition), are metallic conductors on their surfaces but insulators through their interiors. Cadmium arsenide Cd3As2, at about 1 K, is a Dirac-semimetal – a bulk electronic analogue of graphene – in which electrons travel effectively as massless particles. These two classes of material are thought to have potential quantum computing applications.
Nomenclature and history
Derivation and other names
The word metalloid comes from the Latin metallum ("metal") and the Greek oeides ("resembling in form or appearance"). Several names are sometimes used synonymously although some of these have other meanings that are not necessarily interchangeable: amphoteric element, boundary element, half-metal, half-way element, near metal, meta-metal, semiconductor, semimetal and submetal. "Amphoteric element" is sometimes used more broadly to include transition metals capable of forming oxyanions, such as chromium and manganese. "Half-metal" is used in physics to refer to a compound (such as chromium dioxide) or alloy that can act as a conductor and an insulator. "Meta-metal" is sometimes used instead to refer to certain metals (Be, Zn, Cd, Hg, In, Tl, β-Sn, Pb) located just to the left of the metalloids on standard periodic tables. These metals are mostly diamagnetic and tend to have distorted crystalline structures, electrical conductivity values at the lower end of those of metals, and amphoteric (weakly basic) oxides. "Semimetal" sometimes refers, loosely or explicitly, to metals with incomplete metallic character in crystalline structure, electrical conductivity or electronic structure. Examples include gallium, ytterbium, bismuth and neptunium. The names amphoteric element and semiconductor are problematic as some elements referred to as metalloids do not show marked amphoteric behaviour (bismuth, for example) or semiconductivity (polonium) in their most stable forms.
Origin and usage
The origin and usage of the term metalloid is convoluted. Its origin lies in attempts, dating from antiquity, to describe metals and to distinguish between typical and less typical forms. It was first applied in the early 19th century to metals that floated on water (sodium and potassium), and then more popularly to nonmetals. Earlier usage in mineralogy, to describe a mineral having a metallic appearance, can be sourced to as early as 1800. Since the mid-20th century it has been used to refer to intermediate or borderline chemical elements. The International Union of Pure and Applied Chemistry (IUPAC) previously recommended abandoning the term metalloid, and suggested using the term semimetal instead. Use of this latter term has more recently been discouraged by Atkins et al. as it has a different meaning in physics – one that more specifically refers to the electronic band structure of a substance rather than the overall classification of an element. The most recent IUPAC publications on nomenclature and terminology do not include any recommendations on the usage of the terms metalloid or semimetal.
Elements commonly recognised as metalloids
- Properties noted in this section refer to the elements in their most thermodynamically stable forms under ambient conditions.
Boron

Pure boron is a shiny, silver-grey crystalline solid. It is less dense than aluminium (2.34 vs. 2.70 g/cm3), and is hard and brittle. It is barely reactive under normal conditions, except for attack by fluorine, and has a melting point of 2076 °C (cf. steel ~1370 °C). Boron is a semiconductor; its room temperature electrical conductivity is 1.5 × 10−6 S•cm−1 (about 200 times less than that of tap water) and it has a band gap of about 1.56 eV. Mendeleev commented that, "Boron appears in a free state in several forms which are intermediate between the metals and the nonmmetals."
The structural chemistry of boron is dominated by its small atomic size, and relatively high ionization energy. With only three valence electrons per boron atom, simple covalent bonding cannot fulfil the octet rule. Metallic bonding is the usual result among the heavier congenors of boron but this generally requires low ionization energies. Instead, because of its small size and high ionization energies, the basic structural unit of boron (and nearly all of its allotropes) is the icosahedral B12 cluster. Of the 36 electrons associated with 12 boron atoms, 26 reside in 13 delocalized molecular orbitals; the other 10 electrons are used to form two- and three-centre covalent bonds between icosahedra. The same motif can be seen, as are deltahedral variants or fragments, in metal borides and hydride derivatives, and in some halides.
The bonding in boron has been described as being characteristic of behaviour intermediate between metals and nonmetallic covalent network solids (such as diamond). The energy required to transform B, C, N, Si, and P from nonmetallic to metallic states has been estimated as 30, 100, 240, 33, and 50 kJ/mol, respectively. This indicates the proximity of boron to the metal-nonmetal borderline.
Most of the chemistry of boron is nonmetallic in nature. Unlike its heavier congeners, it is not known to form a simple B3+ or hydrated [B(H2O)4]3+ cation. The small size of the boron atom enables the preparation of many interstitial alloy-type borides. Analogies between boron and transition metals have been noted in the formation of complexes, and adducts (for example, BH3 + CO →BH3CO and, similarly, Fe(CO)4 + CO →Fe(CO)5), as well as in the geometric and electronic structures of cluster species such as [B6H6]2− and [Ru6(CO)18]2−. The aqueous chemistry of boron is characterised by the formation of many different polyborate anions. Given its high charge-to-size ratio, boron bonds covalently in nearly all of its compounds; the exceptions are the borides as these include, depending on their composition, covalent, ionic, and metallic bonding components. Simple binary compounds, such as boron trichloride are Lewis acids as the formation of three covalent bonds leaves a hole in the octet which can be filled by an electron-pair donated by a Lewis base. Boron has a strong affinity for oxygen and a duly extensive borate chemistry. The oxide B2O3 is polymeric in structure, weakly acidic, and a glass former. Organometallic compounds of boron have been known since the 19th century (see organoboron chemistry).
Silicon

Silicon is a crystalline solid with a blue-grey metallic lustre. Like boron, it is less dense (at 2.33 g/cm3) than aluminium, and is hard and brittle. It is a relatively unreactive element. According to Rochow, the massive crystalline form (especially if pure) is "remarkably inert to all acids, including hydrofluoric". Less pure silicon, and the powdered form, are variously susceptible to attack by strong or heated acids, as well as by steam and fluorine. Silicon dissolves in hot aqueous alkalis with the evolution of hydrogen, as do metals such as beryllium, aluminium, zinc, gallium or indium. It melts at 1414 °C. Silicon is a semiconductor with an electrical conductivity of 10−4 S•cm−1 and a band gap of about 1.11 eV. When it melts, silicon becomes a reasonable metal with an electrical conductivity of 1.0–1.3 × 104 S•cm−1, similar to that of liquid mercury.
The chemistry of silicon is generally nonmetallic (covalent) in nature. It is not known to form a cation. Silicon can form alloys with metals such as iron and copper. It shows fewer tendencies to anionic behaviour than ordinary nonmetals. Its solution chemistry is characterised by the formation of oxyanions. The high strength of the silicon–oxygen bond dominates the chemical behaviour of silicon. Polymeric silicates, built up by tetrahedral SiO4 units sharing their oxygen atoms, are the most abundant and important compounds of silicon. The polymeric borates, comprising linked trigonal and tetrahedral BO3 or BO4 units, are built on similar structural principles. The oxide SiO2 is polymeric in structure, weakly acidic, and a glass former. Traditional organometallic chemistry includes the carbon compounds of silicon (see organosilicon).
Germanium

Germanium is a shiny grey-white solid. It has a density of 5.323 g/cm3 and is hard and brittle. It is mostly unreactive at room temperature but is slowly attacked by hot concentrated sulfuric or nitric acid. Germanium also reacts with molten caustic soda to yield sodium germanate Na2GeO3 and hydrogen gas. It melts at 938 °C. Germanium is a semiconductor with an electrical conductivity of around 2 × 10−2 S•cm−1 and a band gap of 0.67 eV. Liquid germanium is a metallic conductor, with an electrical conductivity similar to that of liquid mercury.
Most of the chemistry of germanium is characteristic of a nonmetal. Whether or not germanium forms a cation is unclear, aside from the reported existence of the Ge2+ ion in a few esoteric compounds. It can form alloys with metals such as aluminium and gold. It shows fewer tendencies to anionic behaviour than ordinary nonmetals. Its solution chemistry is characterised by the formation of oxyanions. Germanium generally forms tetravalent (IV) compounds, and it can also form less stable divalent (II) compounds, in which it behaves more like a metal. Germanium analogues of all of the major types of silicates have been prepared. The metallic character of germanium is also suggested by the formation of various oxoacid salts. A phosphate [(HPO4)2Ge·H2O] and highly stable trifluoroacetate Ge(OCOCF3)4 have been described, as have Ge2(SO4)2, Ge(ClO4)4 and GeH2(C2O4)3. The oxide GeO2 is polymeric, amphoteric, and a glass former. The dioxide is soluble in acidic solutions (the monoxide GeO, is even more so), and this is sometimes used to classify germanium as a metal. Up to the 1930s germanium was considered to be a poorly conducting metal; it has occasionally been classified as a metal by later writers. As with all the elements commonly recognised as metalloids, germanium has an established organometallic chemistry (see Organogermanium chemistry).
Arsenic

Arsenic is a grey, metallic looking solid. It has a density of 5.727 g/cm3 and is brittle, and moderately hard (more than aluminium; less than iron). It is stable in dry air but develops a golden bronze patina in moist air, which blackens on further exposure. Arsenic is attacked by nitric acid and concentrated sulfuric acid. It reacts with fused caustic soda to give the arsenate Na3AsO3 and hydrogen gas. Arsenic sublimes at 615 °C. The vapour is lemon-yellow and smells like garlic. Arsenic only melts under a pressure of 38.6 atm, at 817 °C. It is a semimetal with an electrical conductivity of around 3.9 × 104 S•cm−1 and a band overlap of 0.5 eV. Liquid arsenic is a semiconductor with a band gap of 0.15 eV.
The chemistry of arsenic is predominately nonmetallic. Whether or not arsenic forms a cation is unclear. Its many metal alloys are mostly brittle. It shows fewer tendencies to anionic behaviour than ordinary nonmetals. Its solution chemistry is characterised by the formation of oxyanions. Arsenic generally forms compounds in which it has an oxidation state of +3 or +5. The halides, and the oxides and their derivatives are illustrative examples. In the trivalent state, arsenic shows some incipient metallic properties. The halides are hydrolysed by water but these reactions, particularly those of the chloride, are reversible with the addition of a hydrohalic acid. The oxide is acidic but, as noted below, (weakly) amphoteric. The higher, less stable, pentavalent state has strongly acidic (nonmetallic) properties. Compared to phosphorus, the stronger metallic character of arsenic is indicated by the formation of oxoacid salts such as AsPO4, As2(SO4)3 and arsenic acetate As(CH3COO)3. The oxide As2O3 is polymeric, amphoteric, and a glass former. Arsenic has an extensive organometallic chemistry (see Organoarsenic chemistry).
Antimony

Antimony is a silver-white solid with a blue tint and a brilliant lustre. It has a density of 6.697 g/cm3 and is brittle, and moderately hard (more so than arsenic; less so than iron; about the same as copper). It is stable in air and moisture at room temperature. It is attacked by concentrated nitric acid, yielding the hydrated pentoxide Sb2O5. Aqua regia gives the pentachloride SbCl5 and hot concentrated sulfuric acid results in the sulfate Sb2(SO4)3. It is not affected by molten alkali. Antimony is capable of displacing hydrogen from water, when heated: 2 Sb + 3 H2O → Sb2O3 + 3 H2. It melts at 631 °C. Antimony is a semimetal with an electrical conductivity of around 3.1 × 104 S•cm−1 and a band overlap of 0.16 eV. Liquid antimony is a metallic conductor with an electrical conductivity of around 5.3 × 104 S•cm−1.
Most of the chemistry of antimony is characteristic of a nonmetal. Antimony has some definite cationic chemistry, SbO+ and Sb(OH)2+ being present in acidic aqueous solution; the compound Sb8(GaCl4)2, which contains the homopolycation, Sb82+, was prepared in 2004. It can form alloys with one or more metals such as aluminium, iron, nickel, copper, zinc, tin, lead, and bismuth. Antimony has fewer tendencies to anionic behaviour than ordinary nonmetals. Its solution chemistry is characterised by the formation of oxyanions. Like arsenic, antimony generally forms compounds in which it has an oxidation state of +3 or +5. The halides, and the oxides and their derivatives are illustrative examples.
The +5 state is less stable than the +3, but relatively easier to
attain than with arsenic. This is explained by the poor shielding
afforded the arsenic nucleus by its 3d10 electrons. In comparison, the tendency of antimony (being a heavier atom) to oxidize more easily partially offsets the effect of its 4d10 shell. Tripositive antimony is amphoteric; pentapositive antimony is (predominately) acidic. Consistent with an increase in metallic character down group 15, antimony forms salts or salt-like compounds including a nitrate Sb(NO3)3, phosphate SbPO4, sulfate Sb2(SO4)3 and perchlorate Sb(ClO4)3. The otherwise acidic pentoxide Sb2O5 shows some basic (metallic) behaviour in that it can be dissolved in very acidic solutions, with the formation of the oxycation SbO+
2. The oxide Sb2O3 is polymeric, amphoteric, and a glass former. Antimony has an extensive organometallic chemistry (see Organoantimony chemistry).
Tellurium

Tellurium is a silvery-white shiny solid. It has a density of 6.24 g/cm3, is brittle, and is the softest of the commonly recognised metalloids, being marginally harder than sulfur. Large pieces of tellurium are stable in air. The finely powdered form is oxidized by air in the presence of moisture. Tellurium reacts with boiling water, or when freshly precipitated even at 50 °C, to give the dioxide and hydrogen: Te + 2 H2O → TeO2 + 2 H2. It reacts (to varying degrees) with nitric, sulfuric, and hydrochloric acids to give compounds such as the sulfoxide TeSO3 or tellurous acid H2TeO3, the basic nitrate (Te2O4H)+(NO3)−, or the oxide sulfate Te2O3(SO4). It dissolves in boiling alkalis, to give the tellurite and telluride: 3 Te + 6 KOH = K2TeO3 + 2 K2Te + 3 H2O, a reaction that proceeds or is reversible with increasing or decreasing temperature.
At higher temperatures tellurium is sufficiently plastic to extrude. It melts at 449.51 °C. Crystalline tellurium has a structure consisting of parallel infinite spiral chains. The bonding between adjacent atoms in a chain is covalent, but there is evidence of a weak metallic interaction between the neighbouring atoms of different chains. Tellurium is a semiconductor with an electrical conductivity of around 1.0 S•cm−1 and a band gap of 0.32 to 0.38 eV. Liquid tellurium is a semiconductor, with an electrical conductivity, on melting, of around 1.9 × 103 S•cm−1. Superheated liquid tellurium is a metallic conductor.
Most of the chemistry of tellurium is characteristic of a nonmetal. It shows some cationic behaviour. The dioxide dissolves in acid to yield the trihydroxotellurium(IV) Te(OH)3+ ion; the red Te42+ and yellow-orange Te62+ ions form when tellurium is oxidized in fluorosulfuric acid (HSO3F), or liquid sulfur dioxide (SO2), respectively. It can form alloys with aluminium, silver, and tin. Tellurium shows fewer tendencies to anionic behaviour than ordinary nonmetals. Its solution chemistry is characterised by the formation of oxyanions. Tellurium generally forms compounds in which it has an oxidation state of −2, +4 or +6. The +4 state is the most stable. Tellurides of composition XxTey are easily formed with most other elements and represent the most common tellurium minerals. Nonstoichiometry is pervasive, especially with transition metals. Many tellurides can be regarded as metallic alloys. The increase in metallic character evident in tellurium, as compared to the lighter chalcogens, is further reflected in the reported formation of various other oxyacid salts, such as a basic selenate 2TeO2·SeO3 and an analogous perchlorate and periodate 2TeO2·HXO4. Tellurium forms a polymeric, amphoteric, glass-forming oxide TeO2. It is a "conditional" glass-forming oxide – it forms a glass with a very small amount of additive. Tellurium has an extensive organometallic chemistry (see Organotellurium chemistry).
Elements less commonly recognised as metalloids
Carbon

Carbon is ordinarily classified as a nonmetal but has some metallic properties and is occasionally classified as a metalloid. Hexagonal graphitic carbon (graphite) is the most thermodynamically stable allotrope of carbon under ambient conditions. It has a lustrous appearance and is a fairly good electrical conductor. Graphite has a layered structure. Each layer consists of carbon atoms bonded to three other carbon atoms in a hexagonal lattice arrangement. The layers are stacked together and held loosely by van der Waals forces and delocalized valence electrons.
Like a metal, the conductivity of graphite in the direction of its planes decreases as the temperature is raised; it has the electronic band structure of a semimetal. The allotropes of carbon, including graphite, can accept foreign atoms or compounds into their structures via substitution, intercalation, or doping. The resulting materials are referred to as "carbon alloys". Carbon can form ionic salts, including a hydrogen sulfate, perchlorate, and nitrate (C+
24X−.2HX, where X = HSO4, ClO4; and C+
24NO–
3.3HNO3). In organic chemistry, carbon can form complex cations – termed carbocations – in which the positive charge is on the carbon atom; examples are CH+
3 and CH+
5, and their derivatives.
Carbon is brittle, and behaves as a semiconductor in a direction perpendicular to its planes. Most of its chemistry is nonmetallic; it has a relatively high ionization energy and, compared to most metals, a relatively high electronegativity. Carbon can form anions such as C4− (methanide), C2–
2 (acetylide), and C3–
4 (sesquicarbide or allylenide), in compounds with metals of main groups 1–3, and with the lanthanides and actinides. Its oxide CO2 forms carbonic acid H2CO3.
Aluminium

Aluminium is ordinarily classified as a metal. It is lustrous, malleable and ductile, and has high electrical and thermal conductivity. Like most metals it has a close-packed crystalline structure, and forms a cation in aqueous solution.
It has some properties that are unusual for a metal; taken together, these are sometimes used as a basis to classify aluminium as a metalloid. Its crystalline structure shows some evidence of directional bonding. Aluminium bonds covalently in most compounds. The oxide Al2O3 is amphoteric and a conditional glass-former. Aluminium can form anionic aluminates, such behaviour being considered nonmetallic in character.
Classifying aluminium as a metalloid has been disputed given its many metallic properties. It is therefore, arguably, an exception to the mnemonic that elements adjacent to the metal–nonmetal dividing line are metalloids.
Stott labels aluminium as a weak metal. It has the physical properties of a metal but some of the chemical properties of a nonmetal. Steele notes the paradoxical chemical behaviour of aluminium: "It resembles a weak metal in its amphoteric oxide and in the covalent character of many of its compounds ... Yet it is a highly electropositive metal ... [with] a high negative electrode potential". Moody says that, "aluminium is on the 'diagonal borderland' between metals and non-metals in the chemical sense."
Selenium

Selenium shows borderline metalloid or nonmetal behaviour.
Its most stable form, the grey trigonal
allotrope, is sometimes called "metallic" selenium because its
electrical conductivity is several orders of magnitude greater than that
of the red monoclinic form. The metallic character of selenium is further shown by its lustre, and its crystalline structure, which is thought to include weakly "metallic" interchain bonding. Selenium can be drawn into thin threads when molten and viscous. It shows reluctance to acquire "the high positive oxidation numbers characteristic of nonmetals". It can form cyclic polycations (such as Se2+
8) when dissolved in oleums
(an attribute it shares with sulfur and tellurium), and a hydrolysed
cationic salt in the form of trihydroxoselenium(IV) perchlorate [Se(OH)3]+·ClO–
4.
The nonmetallic character of selenium is shown by its brittleness and the low electrical conductivity (~10−9 to 10−12 S•cm−1) of its highly purified form. This is comparable to or less than that of bromine (7.95×10–12 S•cm−1), a nonmetal. Selenium has the electronic band structure of a semiconductor and retains its semiconducting properties in liquid form. It has a relatively high electronegativity (2.55 revised Pauling scale). Its reaction chemistry is mainly that of its nonmetallic anionic forms Se2−, SeO2−
3 and SeO2−
4.
Selenium is commonly described as a metalloid in the environmental chemistry literature. It moves through the aquatic environment similarly to arsenic and antimony; its water-soluble salts, in higher concentrations, have a similar toxicological profile to that of arsenic.
Polonium
Polonium is "distinctly metallic" in some ways. Both of its allotropic forms are metallic conductors. It is soluble in acids, forming the rose-coloured Po2+ cation and displacing hydrogen: Po + 2 H+ → Po2+ + H2. Many polonium salts are known. The oxide PoO2 is predominantly basic in nature. Polonium is a reluctant oxidizing agent, unlike its lightest congener oxygen: highly reducing conditions are required for the formation of the Po2− anion in aqueous solution.
Whether polonium is ductile or brittle is unclear. It is predicted to be ductile based on its calculated elastic constants. It has a simple cubic crystalline structure. Such a structure has few slip systems and "leads to very low ductility and hence low fracture resistance".
Polonium shows nonmetallic character in its halides, and by the existence of polonides. The halides have properties generally characteristic of nonmetal halides (being volatile, easily hydrolyzed, and soluble in organic solvents). Many metal polonides, obtained by heating the elements together at 500–1,000 °C, and containing the Po2− anion, are also known.
Astatine
As a halogen, astatine tends to be classified as a nonmetal. It has some marked metallic properties and is sometimes instead classified as either a metalloid or (less often) as a metal. Immediately following its production in 1940, early investigators considered it a metal. In 1949 it was called the most noble (difficult to reduce) nonmetal as well as being a relatively noble (difficult to oxidize) metal. In 1950 astatine was described as a halogen and (therefore) a reactive nonmetal. In 2013, on the basis of relativistic modelling, astatine was predicted to be a monatomic metal, with a face-centred cubic crystalline structure.
Several authors have commented on the metallic nature of some of the properties of astatine. Since iodine is a semiconductor in the direction of its planes, and since the halogens become more metallic with increasing atomic number, it has been presumed that astatine would be a metal if it could form a condensed phase. Astatine may be metallic in the liquid state on the basis that elements with an enthalpy of vaporization (∆Hvap) greater than ~42 kJ/mol are metallic when liquid. Such elements include boron, silicon, germanium, antimony, selenium, and tellurium. Estimated values for ∆Hvap of diatomic astatine are 50 kJ/mol or higher; diatomic iodine, with a ∆Hvap of 41.71, falls just short of the threshold figure.
"Like typical metals, it [astatine] is precipitated by hydrogen sulfide even from strongly acid solutions and is displaced in a free form from sulfate solutions; it is deposited on the cathode on electrolysis." Further indications of a tendency for astatine to behave like a (heavy) metal are: "... the formation of pseudohalide compounds ... complexes of astatine cations ... complex anions of trivalent astatine ... as well as complexes with a variety of organic solvents". It has also been argued that astatine demonstrates cationic behaviour, by way of stable At+ and AtO+ forms, in strongly acidic aqueous solutions.
Some of astatine's reported properties are nonmetallic. It has been extrapolated to have the narrow liquid range ordinarily associated with nonmetals (mp 302 °C; bp 337 °C), although experimental indications suggest a lower boiling point of about 230±3 °C. Batsanov gives a calculated band gap energy for astatine of 0.7 eV; this is consistent with nonmetals (in physics) having separated valence and conduction bands and thereby being either semiconductors or insulators. The chemistry of astatine in aqueous solution is mainly characterised by the formation of various anionic species. Most of its known compounds resemble those of iodine, which is a halogen and a nonmetal. Such compounds include astatides (XAt), astatates (XAtO3), and monovalent interhalogen compounds.
Restrepo et al. reported that astatine appeared to be more polonium-like than halogen-like. They did so on the basis of detailed comparative studies of the known and interpolated properties of 72 elements.
Related concepts
Near metalloids

In the periodic table, some of the elements adjacent to the commonly recognised metalloids, although usually classified as either metals or nonmetals, are occasionally referred to as near-metalloids or noted for their metalloidal character. To the left of the metal–nonmetal dividing line, such elements include gallium, tin and bismuth. They show unusual packing structures, marked covalent chemistry (molecular or polymeric), and amphoterism. To the right of the dividing line are carbon, phosphorus, selenium and iodine. They exhibit metallic lustre, semiconducting properties and bonding or valence bands with delocalized character. This applies to their most thermodynamically stable forms under ambient conditions: carbon as graphite; phosphorus as black phosphorus; and selenium as grey selenium.
Allotropes

Different crystalline forms of an element are called allotropes. Some allotropes, particularly those of elements located (in periodic table terms) alongside or near the notional dividing line between metals and nonmetals, exhibit more pronounced metallic, metalloidal or nonmetallic behaviour than others. The existence of such allotropes can complicate the classification of the elements involved.
Tin, for example, has two allotropes: tetragonal "white" β-tin and cubic "grey" α-tin. White tin is a very shiny, ductile and malleable metal. It is the stable form at or above room temperature and has an electrical conductivity of 9.17 × 104 S·cm−1 (~1/6th that of copper). Grey tin usually has the appearance of a grey micro-crystalline powder, and can also be prepared in brittle semi-lustrous crystalline or polycrystalline forms. It is the stable form below 13.2 °C and has an electrical conductivity of between (2–5) × 102 S·cm−1 (~1/250th that of white tin). Grey tin has the same crystalline structure as that of diamond. It behaves as a semiconductor (as if it had a band gap of 0.08 eV), but has the electronic band structure of a semimetal. It has been referred to as either a very poor metal, a metalloid, a nonmetal or a near metalloid.
The diamond allotrope of carbon is clearly nonmetallic, being translucent and having a low electrical conductivity of 10−14 to 10−16 S·cm−1. Graphite has an electrical conductivity of 3 × 104 S·cm−1, a figure more characteristic of a metal. Phosphorus, sulfur, arsenic, selenium, antimony, and bismuth also have less stable allotropes that display different behaviours.
Abundance, extraction, and cost
| Z | Element | Grams /tonne |
|---|---|---|
| 8 | Oxygen | 461,000 |
| 14 | Silicon | 282,000 |
| 13 | Aluminium | 82,300 |
| 26 | Iron | 56,300 |
| 6 | Carbon | 200 |
| 29 | Copper | 60 |
| 5 | Boron | 10 |
| 33 | Arsenic | 1.8 |
| 32 | Germanium | 1.5 |
| 47 | Silver | 0.075 |
| 34 | Selenium | 0.05 |
| 51 | Antimony | 0.02 |
| 79 | Gold | 0.004 |
| 52 | Tellurium | 0.001 |
| 75 | Rhenium | 7×10−10 |
| 54 | Xenon | 3×10−11 |
| 84 | Polonium | 2×10−16 |
| 85 | Astatine | 3×10−20 |
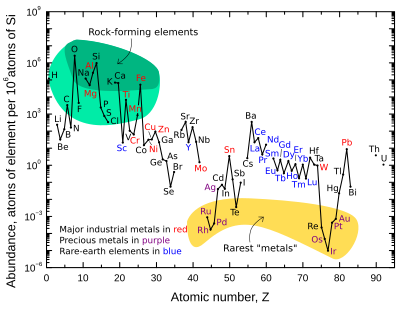
Abundance
The table gives crustal abundances of the elements commonly to rarely recognised as metalloids. Some other elements are included for comparison: oxygen and xenon (the most and least abundant elements with stable isotopes); iron and the coinage metals copper, silver, and gold; and rhenium, the least abundant stable metal (aluminium is normally the most abundant metal). Various abundance estimates have been published; these often disagree to some extent.
Extraction
The recognised metalloids can be obtained by chemical reduction of either their oxides or their sulfides. Simpler or more complex extraction methods may be employed depending on the starting form and economic factors. Boron is routinely obtained by reducing the trioxide with magnesium: B2O3 + 3 Mg → 2 B + 3MgO; after secondary processing the resulting brown powder has a purity of up to 97%. Boron of higher purity (> 99%) is prepared by heating volatile boron compounds, such as BCl3 or BBr3, either in a hydrogen atmosphere (2 BX3 + 3 H2 → 2 B + 6 HX) or to the point of thermal decomposition. Silicon and germanium are obtained from their oxides by heating the oxide with carbon or hydrogen: SiO2 + C → Si + CO2; GeO2 + 2 H2 → Ge + 2 H2O. Arsenic is isolated from its pyrite (FeAsS) or arsenical pyrite (FeAs2) by heating; alternatively, it can be obtained from its oxide by reduction with carbon: 2 As2O3 + 3 C → 2 As + 3 CO2. Antimony is derived from its sulfide by reduction with iron: Sb2S3 → 2 Sb + 3 FeS. Tellurium is prepared from its oxide by dissolving it in aqueous NaOH, yielding tellurite, then by electrolytic reduction: TeO2 + 2 NaOH → Na2TeO3 + H2O; Na2TeO3 + H2O → Te + 2 NaOH + O2.[532] Another option is reduction of the oxide by roasting with carbon: TeO2 + C → Te + CO2.
Production methods for the elements less frequently recognised as metalloids involve natural processing, electrolytic or chemical reduction, or irradiation. Carbon (as graphite) occurs naturally and is extracted by crushing the parent rock and floating the lighter graphite to the surface. Aluminium is extracted by dissolving its oxide Al2O3 in molten cryolite Na3AlF6 and then by high temperature electrolytic reduction. Selenium is produced by roasting the coinage metal selenides X2Se (X = Cu, Ag, Au) with soda ash to give the selenite: X2Se + O2 + Na2CO3 → Na2SeO3 + 2 X + CO2; the selenide is neutralized by sulfuric acid H2SO4 to give selenous acid H2SeO3; this is reduced by bubbling with SO2 to yield elemental selenium. Polonium and astatine are produced in minute quantities by irradiating bismuth.