| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Sulfur dioxide
| |
| Other names
Sulfurous anhydride
Sulfur(IV) oxide | |
| Identifiers | |
3D model (JSmol)
|
|
| 3535237 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.359 |
| EC Number | 231-195-2 |
| E number | E220 (preservatives) |
| 1443 | |
| KEGG | |
| MeSH | Sulfur+dioxide |
PubChem CID
|
|
| RTECS number | WS4550000 |
| UNII | |
| UN number | 1079, 2037 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| SO 2 | |
| Molar mass | 64.066 g mol−1 |
| Appearance | Colorless gas |
| Odor | Pungent; similar to a just-struck match |
| Density | 2.6288 kg m−3 |
| Melting point | −72 °C; −98 °F; 201 K |
| Boiling point | −10 °C (14 °F; 263 K) |
| 94 g/L forms sulfurous acid | |
| Vapor pressure | 237.2 kPa |
| Acidity (pKa) | 1.81 |
| Basicity (pKb) | 12.19 |
| −18.2·10−6 cm3/mol | |
| Viscosity | 0.403 cP (at 0 °C) |
| Structure | |
| C2v | |
| Digonal | |
| Dihedral | |
| 1.62 D | |
| Thermochemistry | |
Std molar
entropy (S |
248.223 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−296.81 kJ mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS signal word | Danger |
| H314, H331 | |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
3000 ppm (mouse, 30 min) 2520 ppm (rat, 1 hr) |
LCLo (lowest published)
|
993 ppm (rat, 20 min) 611 ppm (rat, 5 hr) 764 ppm (mouse, 20 min) 1000 ppm (human, 10 min) 3000 ppm (human, 5 min) |
| US health exposure limits (NIOSH): | |
PEL (Permissible)
|
TWA 5 ppm (13 mg/m3) |
REL (Recommended)
|
TWA 2 ppm (5 mg/m3) ST 5 ppm (13 mg/m3) |
IDLH (Immediate danger)
|
100 ppm |
| Related compounds | |
| Sulfur monoxide Sulfur trioxide | |
Related compounds
|
Ozone
Selenium dioxide
Sulfurous acid
Tellurium dioxide
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfur dioxide (also sulphur dioxide in British English) is the chemical compound with the formula SO
2. It is a toxic gas responsible for the smell of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of the burning of fossil fuels contaminated with sulfur compounds and copper extraction.
Structure and bonding
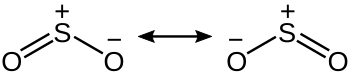
SO2 is a bent molecule with C2v symmetry point group.
A valence bond theory approach considering just s and p orbitals would describe the bonding in terms of resonance between two resonance structures.
Two resonance structures of sulfur dioxide
The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke d orbital participation.
In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1.
Occurrence
The blue auroral glows of Io's upper atmosphere are caused by volcanic sulfur dioxide.
It is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm.
On other planets, it can be found in various concentrations, the most significant being the atmosphere of Venus,
where it is the third-most significant atmospheric gas at 150 ppm.
There, it condenses to form clouds, and is a key component of chemical
reactions in the planet's atmosphere and contributes to global warming. It has been implicated as a key agent in the warming of early Mars, with estimates of concentrations in the lower atmosphere as high as 100 ppm, though it only exists in trace amounts. On both Venus and Mars, as on Earth, its primary source is thought to be volcanic. The atmosphere of Io, a natural satellite of Jupiter, is 90% sulfur dioxide and trace amounts are thought to also exist in the atmosphere of Jupiter.
As an ice, it is thought to exist in abundance on the Galilean moons—as subliming ice or frost on the trailing hemisphere of Io, and in the crust and mantle of Europa, Ganymede, and Callisto, possibly also in liquid form and readily reacting with water.
Production
Sulfur dioxide is primarily produced for sulfuric acid manufacture.
In the United States in 1979, 23.6 million tonnes (26,014,547 US short
tons) of sulfur dioxide were used in this way, compared with 150
thousand tonnes (165,347 US short tons) used for other purposes. Most
sulfur dioxide is produced by the combustion of elemental sulfur. Some
sulfur dioxide is also produced by roasting pyrite and other sulfide ores in air.
Combustion routes
Sulfur dioxide is the product of the burning of sulfur or of burning materials that contain sulfur:
- S + O2 → SO2, ΔH = −297 kJ/mol
To aid combustion, liquefied sulfur (140–150 °C, 284-302 °F) is
sprayed through an atomizing nozzle to generate fine drops of sulfur
with a large surface area. The reaction is exothermic,
and the combustion produces temperatures of 1000–1600 °C,
(1832-2912 °F). The significant amount of heat produced is recovered by
steam generation that can subsequently be converted to electricity.
The combustion of hydrogen sulfide and organosulfur compounds proceeds similarly. For example:
- 2 H2S + 3 O2 → 2 H2O + 2 SO2
The roasting of sulfide ores such as pyrite, sphalerite, and cinnabar (mercury sulfide) also releases SO2:
- 4 FeS2 + 11 O2 → 2 Fe2O3 + 8 SO2
- 2 ZnS + 3 O2 → 2 ZnO + 2 SO2
- HgS + O2 → Hg + SO2
- 4 FeS + 7O2 → 2 Fe2O3 + 4 SO2
A combination of these reactions is responsible for the largest
source of sulfur dioxide, volcanic eruptions. These events can release
millions of tonnes of SO2.
Reduction of higher oxides
Sulfur dioxide can also be a byproduct in the manufacture of calcium silicate cement; CaSO4 is heated with coke and sand in this process:
- 2 CaSO4 + 2 SiO2 + C → 2 CaSiO3 + 2 SO2 + CO2
Until the 1970s, commercial quantities of sulfuric acid and cement were produced by this process in Whitehaven, England. Upon being mixed with shale or marl,
and roasted, the sulfate liberated sulfur dioxide gas, used in sulfuric
acid production, the reaction also produced calcium silicate, a
precursor in cement production.
On a laboratory scale, the action of hot concentrated sulfuric acid on copper turnings produces sulfur dioxide.
- Cu + 2 H2SO4 → CuSO4 + SO2 + 2 H2O
From sulfite
Sulfite results from the reaction of aqueous base and sulfur dioxide. The reverse reaction involves acidification of sodium metabisulfite:
- H2SO4 + Na2S2O5 → 2 SO2 + Na2SO3 + H2O
Reactions
Industrial reactions
Treatment of basic solutions with sulfur dioxide affords sulfite salts (e.g. sodium sulfite):
- SO2 + 2 NaOH → Na2SO3 + H2O
Featuring sulfur in the +4 oxidation state, sulfur dioxide is a reducing agent. It is oxidized by halogens to give the sulfuryl halides, such as sulfuryl chloride:
- SO2 + Cl2 → SO2Cl2
Sulfur dioxide is the oxidising agent in the Claus process, which is conducted on a large scale in oil refineries. Here, sulfur dioxide is reduced by hydrogen sulfide to give elemental sulfur:
- SO2 + 2 H2S → 3 S + 2 H2O
The sequential oxidation of sulfur dioxide followed by its hydration is used in the production of sulfuric acid.
- 2 SO2 + 2 H2O + O2 → 2 H2SO4
Laboratory reactions
Sulfur
dioxide is one of the few common acidic yet reducing gases. It turns
moist litmus pink (being acidic), then white (due to its bleaching
effect). It may be identified by bubbling it through a dichromate solution, turning the solution from orange to green (Cr3+ (aq)). It can also reduce ferric ions to ferrous.
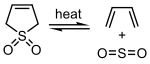
Sulfur dioxide can react with certain 1,3-dienes in a cheletropic reaction to form cyclic sulfones. This reaction is exploited on an industrial scale for the synthesis of sulfolane, which is an important solvent in the petrochemical industry.
Sulfur dioxide can bind to metal ions as a ligand to form metal sulfur dioxide complexes,
typically where the transition metal is in oxidation state 0 or +1.
Many different bonding modes (geometries) are recognized, but in most
cases, the ligand is monodentate, attached to the metal through sulfur,
which can be either planar and pyramidal η1.
Uses
Precursor to sulfuric acid
Sulfur dioxide is an intermediate in the production of sulfuric acid, being converted to sulfur trioxide, and then to oleum,
which is made into sulfuric acid. Sulfur dioxide for this purpose is
made when sulfur combines with oxygen. The method of converting sulfur
dioxide to sulfuric acid is called the contact process. Several billion kilograms are produced annually for this purpose.
As a preservative
Sulfur dioxide is sometimes used as a preservative for dried apricots, dried figs, and other dried fruits, owing to its antimicrobial properties, and is called E220 when used in this way in Europe. As a preservative, it maintains the colorful appearance of the fruit and prevents rotting. It is also added to sulfured molasses.
In winemaking
Sulfur dioxide was first used in winemaking
by the Romans, when they discovered that burning sulfur candles inside
empty wine vessels keeps them fresh and free from vinegar smell.
It is still an important compound in winemaking, and is measured in parts per million (ppm) in wine. It is present even in so-called unsulfurated wine at concentrations of up to 10 mg/L. It serves as an antibiotic and antioxidant,
protecting wine from spoilage by bacteria and oxidation - a phenomenon
that leads to the browning of the wine and a loss of cultivar specific
flavors.
Its antimicrobial action also helps minimize volatile acidity. Wines
containing sulfur dioxide are typically labeled with "containing sulfites".
Sulfur dioxide exists in wine in free and bound forms, and the combinations are referred to as total SO2. Binding, for instance to the carbonyl group of acetaldehyde, varies with the wine in question. The free form exists in equilibrium between molecular SO2
(as a dissolved gas) and bisulfite ion, which is in turn in equilibrium
with sulfite ion. These equilibria depend on the pH of the wine. Lower
pH shifts the equilibrium towards molecular (gaseous) SO2, which is the active form, while at higher pH more SO2 is found in the inactive sulfite and bisulfite forms. The molecular SO2
is active as an antimicrobial and antioxidant, and this is also the
form which may be perceived as a pungent odor at high levels. Wines with
total SO2 concentrations below 10 ppm do not require "contains sulfites" on the label by US and EU laws. The upper limit of total SO2
allowed in wine in the US is 350 ppm; in the EU it is 160 ppm for red
wines and 210 ppm for white and rosé wines. In low concentrations, SO2 is mostly undetectable in wine, but at free SO2 concentrations over 50 ppm, SO2 becomes evident in the smell and taste of wine.
SO2 is also a very important compound in winery
sanitation. Wineries and equipment must be kept clean, and because
bleach cannot be used in a winery due the risk of cork taint, a mixture of SO2, water, and citric acid is commonly used to clean and sanitize equipment. Ozone (O3)
is now used extensively for sanitizing in wineries due to its efficacy,
and because it does not affect the wine or most equipment.
As a reducing agent
Sulfur dioxide is also a good reductant. In the presence of water, sulfur dioxide is able to decolorize substances. Specifically, it is a useful reducing bleach for papers and delicate materials such as clothes. This bleaching effect normally does not last very long. Oxygen
in the atmosphere reoxidizes the reduced dyes, restoring the color. In
municipal wastewater treatment, sulfur dioxide is used to treat
chlorinated wastewater prior to release. Sulfur dioxide reduces free
and combined chlorine to chloride.
Sulfur dioxide is fairly soluble in water, and by both IR and Raman spectroscopy; the hypothetical sulfurous acid, H2SO3, is not present to any extent. However, such solutions do show spectra of the hydrogen sulfite ion, HSO3−, by reaction with water, and it is in fact the actual reducing agent present:
- SO2 + H2O ⇌ HSO3− + H+
Biochemical and biomedical roles
Sulfur
dioxide is toxic in large amounts. It or its conjugate base bisulfite
is produced biologically as an intermediate in both sulfate-reducing
organisms and in sulfur-oxidizing bacteria, as well. The role of sulfur
dioxide in mammalian biology is not yet well understood. Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors and abolishes the Hering–Breuer inflation reflex.
It was shown that endogenous sulfur dioxide plays a role in diminishing an experimental lung damage caused by oleic acid.
Endogenous sulfur dioxide lowered lipid peroxidation, free radical
formation, oxidative stress and inflammation during an experimental lung
damage. Conversely, a successful lung damage caused a significant
lowering of endogenous sulfur dioxide production, and an increase in
lipid peroxidation, free radical formation, oxidative stress and
inflammation. Moreover, blockade of an enzyme that produces endogenous SO2 significantly increased the amount of lung tissue damage in the experiment. Conversely, adding acetylcysteine or glutathione to the rat diet increased the amount of endogenous SO2 produced and decreased the lung damage, the free radical formation, oxidative stress, inflammation and apoptosis.
It is considered that endogenous sulfur dioxide plays a significant physiological role in regulating cardiac and blood vessel
function, and aberrant or deficient sulfur dioxide metabolism can
contribute to several different cardiovascular diseases, such as arterial hypertension, atherosclerosis, pulmonary arterial hypertension, stenocardia.
It was shown that in children with pulmonary arterial hypertension due to congenital heart diseases the level of homocysteine
is higher and the level of endogenous sulfur dioxide is lower than in
normal control children. Moreover, these biochemical parameters strongly
correlated to the severity of pulmonary arterial hypertension. Authors
considered homocysteine to be one of useful biochemical markers of
disease severity and sulfur dioxide metabolism to be one of potential
therapeutic targets in those patients.
Endogenous sulfur dioxide also has been shown to lower the proliferation rate of endothelial smooth muscle cells in blood vessels, via lowering the MAPK activity and activating adenylyl cyclase and protein kinase A. Smooth muscle cell proliferation is one of important mechanisms of hypertensive remodeling of blood vessels and their stenosis, so it is an important pathogenetic mechanism in arterial hypertension and atherosclerosis.
Endogenous sulfur dioxide in low concentrations causes endothelium-dependent vasodilation.
In higher concentrations it causes endothelium-independent vasodilation
and has a negative inotropic effect on cardiac output function, thus
effectively lowering blood pressure and myocardial oxygen consumption.
The vasodilating and bronchodilating effects of sulfur dioxide are
mediated via ATP-dependent calcium channels
and L-type ("dihydropyridine") calcium channels. Endogenous sulfur
dioxide is also a potent antiinflammatory, antioxidant and
cytoprotective agent. It lowers blood pressure and slows hypertensive
remodeling of blood vessels, especially thickening of their intima. It
also regulates lipid metabolism.
Endogenous sulfur dioxide also diminishes myocardial damage, caused by isoproterenol adrenergic hyperstimulation, and strengthens the myocardial antioxidant defense reserve.
As a refrigerant
Being easily condensed and possessing a high heat of evaporation, sulfur dioxide is a candidate material for refrigerants. Prior to the development of chlorofluorocarbons, sulfur dioxide was used as a refrigerant in home refrigerators.
As a reagent and solvent in the laboratory
Sulfur
dioxide is a versatile inert solvent widely used for dissolving highly
oxidizing salts. It is also used occasionally as a source of the
sulfonyl group in organic synthesis. Treatment of aryl diazonium salts with sulfur dioxide and cuprous chloride yields the corresponding aryl sulfonyl chloride, for example:
Proposed use in climate engineering
Injections of sulfur dioxide in the stratosphere has been proposed in climate engineering. The cooling effect would be similar to what has been observed after the large explosive volcano eruption of Mount Pinatubo in 1991. However this form of geoengineering would have uncertain regional consequences on rainfall patterns, for example in monsoon regions.
As an air pollutant
A sulfur dioxide plume from the Halemaʻumaʻu vent, glows at night
Sulfur dioxide is a noticeable component in the atmosphere, especially following volcanic eruptions. According to the United States Environmental Protection Agency, the amount of sulfur dioxide released in the U.S. per year was:
A
collection of estimates of past and future anthropogenic global sulphur
dioxide emissions. The Cofala et al. estimates are for sensitivity
studies on SO2 emission policies, CLE: Current Legislation,
MFR: Maximum Feasible Reductions. RCPs (Representative Concentration
Pathways) are used in CMIP5 simulations for latest (2013–2014) IPCC 5th
assessment report.
| Year | SO2 |
|---|---|
| 1970 | 31,161,000 short tons (28.3 Mt) |
| 1980 | 25,905,000 short tons (23.5 Mt) |
| 1990 | 23,678,000 short tons (21.5 Mt) |
| 1996 | 18,859,000 short tons (17.1 Mt) |
| 1997 | 19,363,000 short tons (17.6 Mt) |
| 1998 | 19,491,000 short tons (17.7 Mt) |
| 1999 | 18,867,000 short tons (17.1 Mt) |
Sulfur dioxide is a major air pollutant and has significant impacts upon human health.
In addition, the concentration of sulfur dioxide in the atmosphere can
influence the habitat suitability for plant communities, as well as
animal life. Sulfur dioxide emissions are a precursor to acid rain and atmospheric particulates. Due largely to the US EPA’s Acid Rain Program, the U.S. has had a 33% decrease in emissions between 1983 and 2002. This improvement resulted in part from flue-gas desulfurization, a technology that enables SO2 to be chemically bound in power plants burning sulfur-containing coal or oil. In particular, calcium oxide (lime) reacts with sulfur dioxide to form calcium sulfite:
- CaO + SO2 → CaSO3
Aerobic oxidation of the CaSO3 gives CaSO4, anhydrite. Most gypsum sold in Europe comes from flue-gas desulfurization.
Sulfur can be removed from coal during burning by using limestone as a bed material in fluidized bed combustion.
Sulfur can also be removed from fuels before burning, preventing formation of SO2 when the fuel is burnt. The Claus process is used in refineries to produce sulfur as a byproduct. The Stretford process has also been used to remove sulfur from fuel. Redox processes using iron oxides can also be used, for example, Lo-Cat or Sulferox.
Sulfur
dioxide in the world on April 15th, 2017. Note that sulfur dioxide
moves through the atmosphere with prevailing winds and thus local sulfur
dioxide distributions vary day to day with weather patterns and
seasonality.
Fuel additives such as calcium
additives and magnesium carboxylate may be used in marine engines to
lower the emission of sulfur dioxide gases into the atmosphere.
As of 2006, China
was the world's largest sulfur dioxide polluter, with 2005 emissions
estimated to be 25,490,000 short tons (23.1 Mt). This amount represents a
27% increase since 2000, and is roughly comparable with U.S. emissions
in 1980.
Safety
US Geological Survey volunteer tests for sulfur dioxide after the 2018 lower Puna eruption
Inhalation
Inhaling
sulfur dioxide is associated with increased respiratory symptoms and
disease, difficulty in breathing, and premature death. In 2008, the American Conference of Governmental Industrial Hygienists reduced the short-term exposure limit to 0.25 parts per million (ppm). The OSHA PEL is currently set at 5 ppm (13 mg/m3) time-weighted average. NIOSH has set the IDLH at 100 ppm. In 2010, the EPA "revised the primary SO2 NAAQS by establishing a new one-hour standard at a level of 75 parts per billion (ppb).
EPA revoked the two existing primary standards because they would not
provide additional public health protection given a one-hour standard at
75 ppb."
A 2011 systematic review concluded that exposure to sulfur dioxide is associated with preterm birth.
Ingestion
In the United States, the Center for Science in the Public Interest lists the two food preservatives, sulfur dioxide and sodium bisulfite, as being safe for human consumption except for certain asthmatic individuals who may be sensitive to them, especially in large amounts. Symptoms of sensitivity to sulfiting agents, including sulfur dioxide, manifest as potentially life-threatening trouble breathing within minutes of ingestion.