The development of the nervous system refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from the nematode and fruit fly to mammals.
Defects in neural development can lead to malformations and a wide
variety of sensory, motor, and cognitive impairments, including holoprosencephaly and other neurological disorders in the human such as Rett syndrome, Down syndrome and intellectual disability.
Overview of brain development
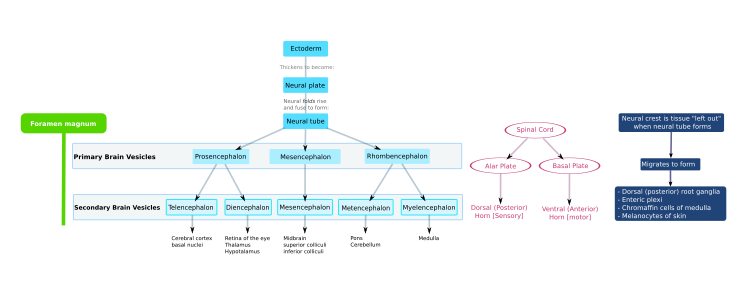
The mammalian central nervous system (CNS) is derived from the ectoderm—the outermost tissue layer—of the embryo. In the third week of human development the neuroectoderm appears and forms the neural plate
along the dorsal side of the embryo. The neural plate is the source of
the majority of neurons and glial cells of the CNS. A groove forms
along the long axis of the neural plate and, by week four of
development, the neural plate wraps in on itself to give rise to the neural tube, which is filled with cerebrospinal fluid (CSF). As the embryo develops, the anterior part of the neural tube forms three brain vesicles, which become the primary anatomical regions of the brain: the forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon). These simple, early vesicles enlarge and further divide into the telencephalon (future cerebral cortex and basal ganglia), diencephalon (future thalamus and hypothalamus), mesencephalon (future colliculi), metencephalon (future pons and cerebellum), and myelencephalon (future medulla). The CSF-filled central chamber is continuous from the telencephalon to the spinal cord, and constitutes the developing ventricular system
of the CNS. Because the neural tube gives rise to the brain and spinal
cord any mutations at this stage in development can lead to fatal
deformities like anencephaly or lifelong disabilities like spina bifida. During this time, the walls of the neural tube contain neural stem cells, which drive brain growth as they divide many times. Gradually some of the cells stop dividing and differentiate into neurons and glial cells, which are the main cellular components of the CNS. The newly generated neurons migrate
to different parts of the developing brain to self-organize into
different brain structures. Once the neurons have reached their regional
positions, they extend axons and dendrites, which allow them to communicate with other neurons via synapses.
Synaptic communication between neurons leads to the establishment of
functional neural circuits that mediate sensory and motor processing,
and underlie behavior.
Flowchart of human brain development.
Aspects
Some landmarks of neural development include the birth and differentiation of neurons from stem cell precursors, the migration of immature neurons from their birthplaces in the embryo to their final positions, outgrowth of axons and dendrites from neurons, guidance of the motile growth cone through the embryo towards postsynaptic partners, the generation of synapses between these axons and their postsynaptic partners, and finally the lifelong changes in synapses, which are thought to underlie learning and memory.
Typically, these neurodevelopmental processes can be broadly
divided into two classes: activity-independent mechanisms and
activity-dependent mechanisms. Activity-independent mechanisms are
generally believed to occur as hardwired processes determined by genetic
programs played out within individual neurons. These include differentiation, migration and axon guidance
to their initial target areas. These processes are thought of as being
independent of neural activity and sensory experience. Once axons
reach their target areas, activity-dependent mechanisms come into play.
Although synapse formation is an activity-independent event,
modification of synapses and synapse elimination requires neural
activity.
Developmental neuroscience uses a variety of animal models including mice Mus musculus, the fruit fly Drosophila melanogaster, the zebrafish Danio rerio, Xenopus laevis tadpoles and the worm Caenorhabditis elegans, among others.
Myelination,
formation of the lipid myelin bilayer around neuronal axons, is a
process that is essential for normal brain function. The myelin sheath
provides insulation for the nerve impulse when communicating between
neural systems. Without it, the impulse would be disrupted and the
signal would not reach its target, thus impairing normal functioning.
Because so much of brain development occurs in the prenatal stage and
infancy, it is crucial that myelination, along with cortical development
occur properly. Magnetic resonance imaging (MRI) is a non-invasive
technique used to investigate myelination and cortical maturation (the
cortex is the outer layer of the brain composed of gray matter).
Rather than showing the actual myelin, the MRI picks up on the myelin
water fraction (MWF), a measure of myelin content. Multicomponent
relaxometry (MCR) allow visualization and quantification of myelin
content. MCR is also useful for tracking white matter maturation, which
plays an important role in cognitive development. It has been discovered
that in infancy, myelination occurs in a posterior-to-anterior pattern.
Because there is little evidence of a relationship between myelination
and cortical thickness, it was revealed that cortical thickness is
independent of white matter MWF. This allows various aspects of the
brain to grow simultaneously, leading to a more fully developed brain.
Neural induction
During
early embryonic development the ectoderm becomes specified to give rise
to the epidermis (skin) and the neural plate. The conversion of
undifferentiated ectoderm to neuro-ectoderm requires signals from the
mesoderm. At the onset of gastrulation presumptive mesodermal cells
move through the dorsal blastopore lip and form a layer in between the
endoderm and the ectoderm. These mesodermal cells that migrate along
the dorsal midline give rise to a structure called the notochord.
Ectodermal cells overlying the notochord develop into the neural plate
in response to a diffusible signal produced by the notochord. The
remainder of the ectoderm gives rise to the epidermis (skin). The
ability of the mesoderm to convert the overlying ectoderm into neural
tissue is called neural induction.
In the human, the neural plate folds outwards during the third week of gestation to form the neural groove. Beginning in the future neck region, the neural folds of this groove close to create the neural tube. The formation of the neural tube from the ectoderm is called neurulation. The ventral part of the neural tube is called the basal plate; the dorsal part is called the alar plate. The hollow interior is called the neural canal. By the end of the fourth week of gestation, the open ends of the neural tube, called the neuropores, close off.
A transplanted blastopore lip can convert ectoderm into neural
tissue and is said to have an inductive effect. Neural inducers are
molecules that can induce the expression of neural genes in ectoderm
explants without inducing mesodermal genes as well. Neural induction is
often studied in xenopus embryos since they have a simple body pattern
and there are good markers to distinguish between neural and non-neural
tissue. Examples of neural inducers are the molecules noggin and chordin.
When embryonic ectodermal cells are cultured at low density in
the absence of mesodermal cells they undergo neural differentiation
(express neural genes), suggesting that neural differentiation is the
default fate of ectodermal cells. In explant cultures (which allow
direct cell-cell interactions) the same cells differentiate into
epidermis. This is due to the action of BMP4 (a TGF-β
family protein) that induces ectodermal cultures to differentiate into
epidermis. During neural induction, noggin and chordin are produced by
the dorsal mesoderm (notochord) and diffuse into the overlying ectoderm
to inhibit the activity of BMP4. This inhibition of BMP4 causes the
cells to differentiate into neural cells. Inhibition of TGF-β and BMP
(bone morphogenetic protein) signaling can efficiently induce neural
tissue from human pluripotent stem cells, a model of early human development.
Regionalization
Late in the fourth week in the human, the superior part of the neural tube flexes at the level of the future midbrain—the mesencephalon. Above the mesencephalon is the prosencephalon (future forebrain) and beneath it is the rhombencephalon (future hindbrain).
The optical vesicle
(which eventually become the optic nerve, retina and iris) forms at the
basal plate of the prosencephalon. The alar plate of the
prosencephalon expands to form the cerebral hemispheres (the telencephalon) whilst its basal plate becomes the diencephalon. Finally, the optic vesicle grows to form an optic outgrowth.
Patterning of the nervous system
In chordates,
dorsal ectoderm forms all neural tissue and the nervous system.
Patterning occurs due to specific environmental conditions - different
concentrations of signaling molecules
Dorsoventral axis
The ventral half of the neural plate is controlled by the notochord, which acts as the 'organiser'. The dorsal half is controlled by the ectoderm plate, which flanks either side of the neural plate.
Ectoderm follows a default pathway to become neural tissue.
Evidence for this comes from single, cultured cells of ectoderm, which
go on to form neural tissue. This is postulated to be because of a lack
of BMPs, which are blocked by the organiser. The organiser may produce molecules such as follistatin, noggin and chordin that inhibit BMPs.
The ventral neural tube is patterned by sonic hedgehog (Shh) from the notochord, which acts as the inducing tissue. Notochord-derived Shh signals to the floor plate,
and induces Shh expression in the floor plate. Floor plate-derived Shh
subsequently signals to other cells in the neural tube, and is
essential for proper specification of ventral neuron progenitor domains.
Loss of Shh from the notochord and/or floor plate prevents proper
specification of these progenitor domains. Shh binds Patched1, relieving Patched-mediated inhibition of Smoothened, leading to activation of the Gli family of transcription factors (Gli1, Gli2, and Gli3).
In this context Shh acts as a morphogen
- it induces cell differentiation dependent on its concentration. At
low concentrations it forms ventral interneurones, at higher
concentrations it induces motor neuron development, and at highest
concentrations it induces floor plate differentiation. Failure of
Shh-modulated differentiation causes holoprosencephaly.
The dorsal neural tube is patterned by BMPs from the epidermal
ectoderm flanking the neural plate. These induce sensory interneurones
by activating Sr/Thr kinases and altering SMAD transcription factor levels.
Rostrocaudal (Anteroposterior) axis
Signals that control anteroposterior neural development include FGF and retinoic acid, which act in the hindbrain and spinal cord. The hindbrain, for example, is patterned by Hox genes,
which are expressed in overlapping domains along the anteroposterior
axis under the control of retinoic acid. The 3' genes in the Hox cluster
are induced by retinoic acid in the hindbrain, whereas the 5' Hox genes
are not induced by retinoic acid and are expressed more posteriorly in
the spinal cord. Hoxb-1 is expressed in rhombomere 4 and gives rise to
the facial nerve. Without this Hoxb-1 expression, a nerve similar to the trigeminal nerve arises.
Neurogenesis
Neurogenesis is the process by which neurons are generated from neural stem cells and progenitor cells. Neurons are 'post-mitotic', meaning that they will never divide again for the lifetime of the organism.
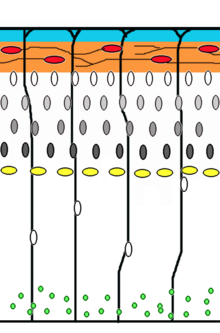
Neuronal migration
Corticogenesis: younger neurons migrate past older ones using radial glia as a scaffolding. Cajal-Retzius cells (red) release reelin (orange).
Neuronal migration
is the method by which neurons travel from their origin or birthplace
to their final position in the brain. There are several ways they can do
this, e.g. by radial migration or tangential migration. This time lapse displays sequences of radial migration (also known as glial guidance) and somal translocation.
Tangential migration of interneurons from ganglionic eminence.
Radial migration
Neuronal precursor cells proliferate in the ventricular zone of the developing neocortex, where the principal neural stem cell is the radial glial cell. The first postmitotic cells must leave the stem cell niche and migrate outward to form the preplate, which is destined to become Cajal-Retzius cells and subplate
neurons. These cells do so by somal translocation. Neurons migrating
with this mode of locomotion are bipolar and attach the leading edge of
the process to the pia. The soma is then transported to the pial surface by nucleokinesis, a process by which a microtubule "cage" around the nucleus elongates and contracts in association with the centrosome to guide the nucleus to its final destination. Radial glial cells,
whose fibers serve as a scaffolding for migrating cells and a means of
radial communication mediated by calcium dynamic activity, act as the main excitatory neuronal stem cell of the cerebral cortex or translocate to the cortical plate and differentiate either into astrocytes or neurons. Somal translocation can occur at any time during development.
Subsequent waves of neurons split the preplate by migrating along radial glial
fibres to form the cortical plate. Each wave of migrating cells travel
past their predecessors forming layers in an inside-out manner, meaning
that the youngest neurons are the closest to the surface. It is estimated that glial guided migration represents 90% of migrating neurons in human and about 75% in rodents.
Tangential migration
Most
interneurons migrate tangentially through multiple modes of migration
to reach their appropriate location in the cortex. An example of
tangential migration is the movement of interneurons from the ganglionic eminence to the cerebral cortex. One example of ongoing tangential migration in a mature organism, observed in some animals, is the rostral migratory stream connecting subventricular zone and olfactory bulb.
Axophilic migration
Many neurons migrating along the anterior-posterior axis of the body use existing axon tracts to migrate along; this is called axophilic migration. An example of this mode of migration is in GnRH-expressing neurons, which make a long journey from their birthplace in the nose, through the forebrain, and into the hypothalamus. Many of the mechanisms of this migration have been worked out, starting with the extracellular guidance cues that trigger intracellular signaling. These intracellular signals, such as calcium signaling, lead to actin and microtubule cytoskeletal dynamics, which produce cellular forces that interact with the extracellular environment through cell adhesion proteins to cause the movement of these cells.
Other modes of migration
There is also a method of neuronal migration called multipolar migration. This is seen in multipolar cells, which in the human, are abundantly present in the cortical intermediate zone.
They do not resemble the cells migrating by locomotion or somal
translocation. Instead these multipolar cells express neuronal markers
and extend multiple thin processes in various directions independently
of the radial glial fibers.
Neurotrophic factors
The
survival of neurons is regulated by survival factors, called trophic
factors. The neurotrophic hypothesis was formulated by Victor Hamburger
and Rita Levi Montalcini
based on studies of the developing nervous system. Victor Hamburger
discovered that implanting an extra limb in the developing chick led to
an increase in the number of spinal motor neurons. Initially he thought
that the extra limb was inducing proliferation of motor neurons, but he
and his colleagues later showed that there was a great deal of motor
neuron death during normal development, and the extra limb prevented
this cell death. According to the neurotrophic hypothesis, growing axons
compete for limiting amounts of target-derived trophic factors and
axons that fail to receive sufficient trophic support die by apoptosis.
It is now clear that factors produced by a number of sources contribute
to neuronal survival.
- Nerve Growth Factor (NGF): Rita Levi Montalcini and Stanley Cohen purified the first trophic factor, Nerve Growth Factor (NGF), for which they received the Nobel Prize. There are three NGF-related trophic factors: BDNF, NT3, and NT4, which regulate survival of various neuronal populations. The Trk proteins act as receptors for NGF and related factors. Trk is a receptor tyrosine kinase. Trk dimerization and phosphorylation leads to activation of various intracellular signaling pathways including the MAP kinase, Akt, and PKC pathways.
- CNTF: Ciliary neurotrophic factor is another protein that acts as a survival factor for motor neurons. CNTF acts via a receptor complex that includes CNTFRα, GP130, and LIFRβ. Activation of the receptor leads to phosphorylation and recruitment of the JAK kinase, which in turn phosphorylates LIFRβ. LIFRβ acts as a docking site for the STAT transcription factors. JAK kinase phosphorylates STAT proteins, which dissociate from the receptor and translocate to the nucleus to regulate gene expression.
- GDNF: Glial derived neurotrophic factor is a member of the TGFb family of proteins, and is a potent trophic factor for striatal neurons. The functional receptor is a heterodimer, composed of type 1 and type 2 receptors. Activation of the type 1 receptor leads to phosphorylation of Smad proteins, which translocate to the nucleus to activate gene expression.
Synapse formation
Neuromuscular junction
Much of our understanding of synapse formation comes from studies at
the neuromuscular junction. The transmitter at this synapse is
acetylcholine. The acetylcholine receptor (AchR) is present at the
surface of muscle cells before synapse formation. The arrival of the
nerve induces clustering of the receptors at the synapse. McMahan and
Sanes showed that the synaptogenic signal is concentrated at the basal lamina. They also showed that the synaptogenic signal is produced by the nerve, and they identified the factor as Agrin.
Agrin induces clustering of AchRs on the muscle surface and synapse
formation is disrupted in agrin knockout mice. Agrin transduces the
signal via MuSK receptor to rapsyn.
Fischbach and colleagues showed that receptor subunits are selectively
transcribed from nuclei next to the synaptic site. This is mediated by
neuregulins.
In the mature synapse each muscle fiber is innervated by one
motor neuron. However, during development many of the fibers are
innervated by multiple axons. Lichtman and colleagues have studied the
process of synapses elimination. This is an activity-dependent event.
Partial blockage of the receptor leads to retraction of corresponding
presynaptic terminals.
CNS synapses
Agrin
appears not to be a central mediator of CNS synapse formation and there
is active interest in identifying signals that mediate CNS
synaptogenesis. Neurons in culture develop synapses that are similar to
those that form in vivo, suggesting that synaptogenic signals can
function properly in vitro. CNS synaptogenesis studies have focused
mainly on glutamatergic synapses. Imaging experiments show that
dendrites are highly dynamic during development and often initiate
contact with axons. This is followed by recruitment of postsynaptic
proteins to the site of contact. Stephen Smith and colleagues have shown
that contact initiated by dendritic filopodia can develop into synapses.
Induction of synapse formation by glial factors: Barres and
colleagues made the observation that factors in glial conditioned media
induce synapse formation in retinal ganglion cell cultures. Synapse
formation in the CNS is correlated with astrocyte differentiation
suggesting that astrocytes might provide a synaptogenic factor. The
identity of the astrocytic factors is not yet known.
Neuroligins and SynCAM as synaptogenic signals: Sudhof, Serafini,
Scheiffele and colleagues have shown that neuroligins and SynCAM can
act as factors that induce presynaptic differentiation. Neuroligins are
concentrated at the postsynaptic site and act via neurexins concentrated
in the presynaptic axons. SynCAM is a cell adhesion molecule that is
present in both pre- and post-synaptic membranes.
Activity dependent mechanisms in the assembly of neural circuits
The processes of neuronal migration, differentiation and axon guidance
are generally believed to be activity-independent mechanisms and rely
on hard-wired genetic programs in the neurons themselves. New research
findings however have implicated a role for activity-dependent
mechanisms in mediating some aspects of the aforementioned processes
such as the rate of neuronal migration, aspects of neuronal differentiation and axon pathfinding.
Activity-dependent mechanisms influence neural circuit development and
are crucial for laying out early connectivity maps and the continued
refinement of synapses which occurs during development.
There are two distinct types of neural activity we observe in
developing circuits -early spontaneous activity and sensory-evoked
activity. Spontaneous activity occurs early during neural circuit development even when sensory input is absent and is observed in many systems such as the developing visual system, auditory system, motor system, hippocampus, cerebellum, and neocortex.
Experimental techniques such as direct electrophysiological
recording, fluorescence imaging using calcium indicators and optogenetic
techniques have shed light on the nature and function of these early
bursts of activity. They have distinct spatial and temporal patterns during development and their ablation during development has been known to result in deficits in network refinement in the visual system. In the immature retina, waves of spontaneous action potentials arise from the retinal ganglion cells and sweep across the retinal surface in the first few postnatal weeks. These waves are mediated by neurotransmitter acetylcholine in the initial phase and later on by glutamate. They are thought to instruct the formation of two sensory maps- the retinotopic map and eye-specific segregation. Retinotopic map refinement occurs in downstream visual targets in the brain-the superior colliculus (SC) and dorsal lateral geniculate nucleus (LGN). Pharmacological disruption and mouse models lacking the β2 subunit of the nicotinic acetylcholine receptor has shown that the lack of spontaneous activity leads to marked defects in retinotopy and eye-specific segregation.
In the developing auditory system, developing cochlea generate bursts of activity which spreads across the inner hair cells and spiral ganglion neurons which relay auditory information to the brain. ATP release from supporting cells triggers action potentials in inner hair cells.
In the auditory system, spontaneous activity is thought to be involved
in tonotopic map formation by segregating cochlear neuron axons tuned to
high and low frequencies. In the motor system, periodic bursts of spontaneous activity are driven by excitatory GABA and glutamate during the early stages and by acetylcholine and glutamate at later stages. In the developing zebrafish spinal cord,
early spontaneous activity is required for the formation of
increasingly synchronous alternating bursts between ipsilateral and
contralateral regions of the spinal cord and for the integration of new
cells into the circuit. In the cortex, early waves of activity have been observed in the cerebellum and cortical slices.
Once sensory stimulus becomes available, final fine-tuning of
sensory-coding maps and circuit refinement begins to rely more and more
on sensory-evoked activity as demonstrated by classic experiments about
the effects of sensory deprivation during critical periods.
Contemporary diffusion-weigthted MRI techniques may also uncover the macroscopic process of axonal development. The connectome can be constructed from diffusion MRI data: the vertices of the graph correspond to anatomically labelled gray matter areas, and two such vertices, say u and v, are connected by an edge if the tractography phase of the data processing finds an axonal fiber that connects the two areas, corresponding to u and v.
Numerous braingraphs, computed from the Human Connectome Project can be downloaded from the http://braingraph.org
site. The Consensus Connectome Dynamics (CCD) is a remarkable
phenomenon that was discovered by continuously decreasing the minimum
confidence-parameter at the graphical interface of the Budapest Reference Connectome Server. The Budapest Reference Connectome Server (http://connectome.pitgroup.org)
depicts the cerebral connections of n=418 subjects with a
frequency-parameter k: For any k=1,2,...,n one can view the graph of the
edges that are present in at least k connectomes. If parameter k is
decreased one-by-one from k=n through k=1 then more and more edges
appear in the graph, since the inclusion condition is relaxed. The
surprising observation is that the appearance of the edges is far from
random: it resembles a growing, complex structure, like a tree or a
shrub (visualized on the animation on the left).
It is hypothesized in that the growing structure copies the axonal development of the human brain:
the earliest developing connections (axonal fibers) are common at most
of the subjects, and the subsequently developing connections have larger
and larger variance, because their variances are accumulated in the
process of axonal development.
Synapse elimination
Several motorneurons compete for each neuromuscular junction, but only one survives until adulthood. Competition in vitro
has been shown to involve a limited neurotrophic substance that is
released, or that neural activity infers advantage to strong
post-synaptic connections by giving resistance to a toxin also released
upon nerve stimulation. In vivo, it is suggested that muscle fibres select the strongest neuron through a retrograde signal.
Adult neurogenesis
Contrary to popular belief, neurogenesis also occurs in specific parts of the adult brain.