Solar still built into a pit in the ground
"Watercone" solar still
A solar still distills water with substances dissolved in it by using the heat of the Sun
to evaporate water so that it may be cooled and collected, thereby
purifying it. They are used in areas where drinking water is
unavailable, so that clean water is obtained from dirty water or from
plants by exposing them to sunlight.
There are many types of solar still, including large scale concentrated solar stills and condensation traps (better known as moisture traps amongst survivalists).
In a solar still, impure water is contained outside the collector,
where it is evaporated by sunlight shining through clear plastic or
glass. The pure water vapour condenses on the cool inside surface and drips down, where it is collected and removed.
Distillation replicates the way nature makes rain. The sun's
energy heats water to the point of evaporation. As the water evaporates,
water vapour rises, condensing into water again as it cools and can
then be collected. This process leaves behind impurities, such as salts
and heavy metals, and eliminates microbiological organisms. The end
result is pure distilled drinking water.
History
Today, a method for gathering water in moisture traps is still taught within the Argentinian Army
for use by specialist units expected to conduct extended patrols of
more than a week's duration in the arid border areas of the Andes.
Uses
Solar stills are used in cases where rain, piped, or well water is impractical, such as in remote homes or during power outages. In subtropical hurricane target areas that can lose power for days, solar distillation can provide an alternative source of clean water.
Solar Well
Methods
Several methods of trapping condensation exist:
First method
This
method was first used by the peoples of the Andes. A pit is dug into
the earth, at the bottom of which is placed the receptacle that will be
used to catch the condensed water. Small branches are placed with one of
their ends inside the receptacle and their other ends up over the edge
of the pit, forming a funnel to direct the condensed water into the
receptacle. A lid is then built over this funnel, using more small
branches, leaves, grasses, etc. The completed trap is left overnight,
and moisture can be collected from the receptacle in the morning.
This method relies on the formation of dew or frost
on the receptacle, funnel, and lid. Forming dew collects on and runs
down the outside of the funnel and into the receptacle. This water would
typically evaporate with the morning sun and thus vanish, but the lid traps the evaporating water and raises the humidity
within the trap, reducing the amount of water that is lost. The shade
produced by the lid also reduces the temperature within the trap, which
further reduces the rate of water loss to evaporation.
Modern method
Today, with the advent of plastic sheeting, the moisture trap has become more efficient.
The method is very similar to that described above, but a single
sheet of plastic is used instead of branches and leaves. The greater
efficiency of this type of trap arises from the waterproof nature of the
plastic, which doesn't let any water vapour pass through it (some water
vapour escapes through the leaves and branches of the first method).
This efficiency requires a certain amount of diligence of the part of
the user, in that the plastic sheet must be firmly attached to the
ground on all sides; this is often accomplished by using stones to
weight the sheet down and/or covering the edges of the plastic sheet
with earth (such as that dug out to make the hole in which the trap
sits). Weighting the centre of the plastic sheet down with a stone forms
the funnel via which the condensed water will run into the receptacle.
Transpiration method
Water can be obtained by placing clear plastic bags over the leafy branch of a non-poisonous tree and tightly closing the bag's open end around the branch. Any holes in the bag must be sealed to prevent the loss of water vapour.
During photosynthesis plants lose water through a process called transpiration. A clear plastic bag sealed around a branch allows photosynthesis to continue, but traps the evaporating water causing the vapour pressure
of water to rise to a point where it begins to condense on the surface
of the plastic bag. Gravity then causes the water to run to the lowest
part of the bag. Water is collected by tapping
the bag and then resealing it. The leaves will continue to produce
water as the roots draw it from the ground and photosynthesis occurs.
The vapour pressure of water in the sealed bag can rise so high
that the leaves can no longer transpire, consequently when using this
method, the water should be drained off every two hours and stored.
Tests indicate that if this is not done the leaves stop producing water.
If there are no large trees in the area, clumps of grass or small
bushes can be placed inside the bag. If this is done the foliage will
have to be replaced at regular intervals when water production is
reduced, particularly if the foliage must be uprooted to place it in the
bag.
Efficiency is greatest when the bag receives maximum sunshine at
all times. Exposed roots are tested for water content. Soft, pulpy roots
will yield the greatest amount of liquid for the least amount of
effort.
Condensation trap efficiency
Condensation
traps are not in themselves a sustainable source of water; they are
sources for extending or supplementing existing water sources or
supplies, and should not be relied on to provide a person's daily
requirement for water, since a trap measuring 400 mm (16 in) in diameter
by 300 mm (12 in) deep will only yield around 100 to 150 mL (3.4 to
5.1 US fl oz) per day.
One method to increase the water output is to urinate
into the pit before placing the receptacle in. This increases the
moisture content of the earth, reducing the amount of water vapour that
the earth can subsequently absorb.
Materials
A simple basin-type solar still can be constructed with 2–4 stones, plastic film or transparent glass,
a central weight to make a point and a container for the condensate. A
cubic hole in moist ground is created of about 300 mm (12 in) on each
side. Into the centre of this hole, a collection container is placed.
Then a sheet of plastic film is stretched over the hole. Stills can also
be made from water bottles or plastic bags.
Variations
Transpiration bag
An alternative method of the solar still is called the transpiration bag.
The bag is a simple plastic bag and it folds over a stemmed plant with a
corner pointing down to allow the condensate to pool. From there a
person can remove the water by taking the bag off and pouring the water
out or one can make a tiny incision into the corner to drip water into a
cup. Its advantage over the basin type solar still mentioned before is
that it only requires a bag like one can get at the grocery store. It
doesn't need to be completely transparent. A disadvantage of the
transpiration bag is the requirement for a plant in direct sunlight or
heat to take the condensate.
In a study performed in 2009,
variations to the angle of plastic and increasing the internal
temperature of the hole versus the outside temperature made for better
water production. Other methods used included using a brine to absorb
water from and adding dyes to the brine
to change the amount of solar radiation absorbed into the system.
During the adjusted tilt angle experiment, the different angles used by
the different researchers created different results and it was difficult
for any of them to get a definite answer. In the graph, a bell curve is
observed with the maximum water output being at 30 degrees angle
adjustment. Each brine depth created a different amount of water and it
is noted on the graph that about 25 millimetres (1 in) is optimal with a
decreasing trend if more is used.
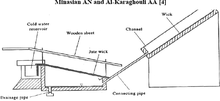
Wick still
This image shows how a wick basin solar still works.
The “wick” type solar still is a glass-topped box constructed and held at angle to allow sunlight in.
Salt water poured in from the top is heated by sunlight, evaporating
the water. It condenses on the underside of the glass and drips to the
bottom. A pool of brine in the still is attached to the wicks which
separates the water into banks to increase surface area for heating. The
distilled water comes out of the bottom and, depending on the quality
of construction, most of the salt has been purged from the water. The
more wicks, the more heat can be transferred to the salt water and more
product can be made. A plastic net can also catch salt water before it
falls into the container and give it more time to heat up and separate
into brine and water. The wick type solar still is made vapour-tight, as
in the vapour does not escape to the atmosphere. To aid in absorbing
more heat, some wicks are blackened to take in more heat. Glass's
absorption of heat is negligible compared to plastic at higher
temperatures. A problem, depending on application, with glass is that it
is not flexible if the solar still is not a standard shape.
Practical considerations
The pit still may be inefficient as a survival still, requiring too much construction effort for the water produced.
In desert environments water needs can exceed 3.8 litres (1 US gal) per
day for a person at rest, while still production may average 240
millilitres (8 US fl oz) per day. Even with tools, digging a hole requires energy and makes a person lose water through perspiration; this means that even several days of water collection may not be equal to the water lost in its construction.
Seawater still
In
1952, the United States military developed a portable solar still for
pilots stranded on the ocean, which comprises an inflatable
610-millimetre (24 in) plastic ball that floats on the ocean, with a
flexible tube coming out the side. A separate plastic bag hangs from
attachment points on the outer bag. Seawater is poured into the inner
bag from an opening in the ball's neck. Fresh water is taken out by the
pilot using the side tube that leads to bottom of the inflatable ball.
It was stated in magazine articles that on a good day 2.4 litres
(2.5 US qt) of fresh water could be produced. On an overcast day, 1.4
litres (1.5 US qt) was produced. Similar sea water stills are included in some life raft survival kits, though manual reverse osmosis desalinators have mostly replaced them.
Distilling urine
Using a condensation trap to distill urine will remove the urea and salt, providing one with drinkable water as a result.