Gender-affirming hormone therapy (GAHT), also called hormone replacement therapy (HRT) or transgender hormone therapy, is a form of hormone therapy in which sex hormones and other hormonal medications are administered to transgender or gender nonconforming individuals for the purpose of more closely aligning their secondary sexual characteristics with their gender identity. This form of hormone therapy is given as one of two types, based on whether the goal of treatment is masculinization or feminization:
- Masculinizing hormone therapy – for transgender men or transmasculine people; consists of androgens and occasionally antiestrogens.
- Feminizing hormone therapy – for transgender women or transfeminine people; consists of estrogens with or without antiandrogens.
Eligibility for GAHT may require an assessment for gender dysphoria or persistent gender incongruence; or many medical institutions now use an informed consent model, which ensures patients are informed of the procedure process, including possible benefits and risks, while removing many of the historical barriers needed to start hormone therapy. Treatment guidelines for therapy have been developed by several medical associations.
Non-binary people may also engage in hormone therapy in order to achieve a desired balance of sex hormones or to help align their bodies with their gender identities. Many transgender people obtain hormone therapy from a licensed health care provider and others obtain and self-administer hormones.
History
Requirements
The formal requirements to begin gender-affirming hormone therapy vary widely depending on geographic location and specific institution. Gender-affirming hormones can be prescribed by a wide range of medical providers including, but not limited to, primary care physicians, endocrinologists, and gynecologists. Requirements generally include a minimum age; according to the Endocrine Society, there has been little research on taking cross-sex hormones before the age of about 14.
Historically, many health centers required a psychiatric evaluation and/or a letter from a therapist before beginning therapy. Many centers now use an informed consent model that does not require any routine formal psychiatric evaluation but instead focuses on reducing barriers to care by ensuring a person can understand the risks, benefits, alternatives, unknowns, limitations, and risks of no treatment. Some LGBT health organizations (notably Chicago's Howard Brown Health Center and Planned Parenthood) advocate for this type of informed consent model.
The World Professional Association for Transgender Health (WPATH) Standards of Care, 7th edition, note that both of these approaches to care are appropriate.
Gender dysphoria
Many international guidelines and institutions require persistent, well-documented gender dysphoria as a pre-requisite to starting gender-affirmation therapy. Gender dysphoria refers to the psychological discomfort or distress that an individual can experience if their sex assigned at birth is incongruent with that person's gender identity. Signs of gender dysphoria can include comorbid mental health stressors such as depression, anxiety, low self-esteem, and social isolation. Not all gender nonconforming individuals experience gender dysphoria, and measuring a person's gender dysphoria is critical when considering medical intervention for gender nonconformity.
Treatment options
Guidelines
For transgender youth, the Dutch protocol existed as among the earlier guidelines for hormone therapy by delaying puberty until age 16. The World Professional Association for Transgender Health (WPATH) and the Endocrine Society later formulated guidelines that created a foundation for health care providers to care for transgender patients. UCSF guidelines are also sometimes used. There is no generally agreed-upon set of guidelines, however.
Delaying puberty in adolescents
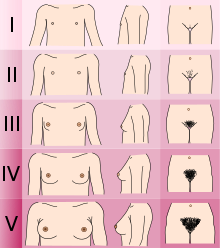
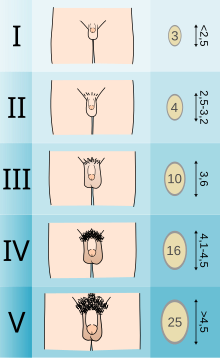
Adolescents experiencing gender dysphoria may opt to undergo puberty-suppressing hormone therapy at the onset of puberty. The Standards of Care set forth by WPATH recommend individuals pursuing puberty-suppressing hormone therapy wait until at least experiencing Tanner Stage 2 pubertal development. Tanner Stage 2 is defined by the appearance of scant pubic hair, breast bud development, and/or slight testicular growth. WPATH classifies puberty-suppressing hormone therapy as a "fully reversible" intervention. Delaying puberty allows individuals more time to explore their gender identity before deciding on more permanent interventions and prevents the physical changes associated with puberty.
The preferred puberty-suppressing agent for both individuals assigned male at birth and individuals assigned female at birth is a GnRH Analogue. This approach temporarily shuts down the Hypothalamic-Pituitary-Gonadal (HPG) Axis, which is responsible for the production of hormones (estrogen, testosterone) that cause the development of secondary sexual characteristics in puberty.
Feminizing hormone therapy
Feminizing hormone therapy is typically used by transgender women, who desire the development of feminine secondary sex characteristics. Individuals who identify as non-binary may also opt-in for feminizing hormone treatment to better align their body with their desired gender expression. Feminizing hormone therapy usually includes medication to suppress testosterone production and induce feminization. Types of medications include estrogens, antiandrogens (testosterone blockers), and progestogens. Most commonly, an estrogen is combined with an antiandrogen to suppress and block testosterone. This allows for demasculinization and promotion of feminization and breast development. Estrogens are administered in various modalities including injection, transdermal patch, and oral tablets.
The desired effects of feminizing hormone therapy focus on the development of feminine secondary sex characteristics. These desired effects include: breast tissue development, redistribution of body fat, decreased body hair, reduction of muscle mass, and more. The table below summarizes some of the effects of feminizing hormone therapy in transgender women:
| Effect | Time to expected onset of effect |
Time to expected maximum effect |
Permanency if hormone therapy is stopped |
|---|---|---|---|
| Breast development and nipple/areolar enlargement | 2–6 months | 1–5 years | Permanent |
| Thinning/slowed growth of facial/body hair | 4–12 months | >3 years | Reversible |
| Cessation/reversal of male-pattern scalp hair loss | 1–3 months | 1–2 years | Reversible |
| Softening of skin/decreased oiliness and acne | 3–6 months | Unknown | Reversible |
| Redistribution of body fat in a feminine pattern | 3–6 months | 2–5 years | Reversible |
| Decreased muscle mass/strength | 3–6 months | 1–2 years | Reversible |
| Widening and rounding of the pelvis | Unspecified | Unspecified | Permanent |
| Changes in mood, emotionality, and behavior | Unspecified | Unspecified | Reversible |
| Decreased sex drive | 1–3 months | Temporary | Reversible |
| Decreased spontaneous/morning erections | 1–3 months | 3–6 months | Reversible |
| Erectile dysfunction and decreased ejaculate volume | 1–3 months | Variable | Reversible |
| Decreased sperm production/fertility | Unknown | >3 years | Reversible or permanent |
| Decreased testicle size | 3–6 months | 2–3 years | Unknown |
| Decreased penis size | None | Not applicable | Not applicable |
| Decreased prostate gland size | Unspecified | Unspecified | Unspecified |
| Voice changes | None | Not applicable | Not applicable |
|
Masculinizing hormone therapy
Masculinizing hormone therapy is typically used by transgender men, who desire the development of masculine secondary sex characteristics. Masculinizing hormone therapy usually includes testosterone to produce masculinization and suppress the production of estrogen. Treatment options include oral, subcutaneous injections or implant, and transdermal (patches, gels). Dosing is patient-specific, depending on the patient's rate of metabolism, and is discussed with the physician. The most commonly prescribed methods are intramuscular and subcutaneous injections. This dosing can be daily, weekly or biweekly depending on the route of administration and the individual patient.
Unlike feminizing hormone therapy, individuals undergoing masculinizing hormone therapy do not usually require additional hormone suppression such as estrogen suppression. Therapeutic doses of testosterone are usually sufficient to inhibit the production of estrogen to desired physiologic levels.
The desired effects of masculinizing hormone therapy focus on the development of masculine secondary sex characteristics. These desired effects include: increased muscle mass, development of facial hair, voice deepening, increase and thickening of body hair, and more.
| Reversible Changes | Irreversible Changes |
|---|---|
| Increased libido | Deepening of voice |
| Redistribution of body fat | Growth of facial/body hair |
| Cessation of ovulation/menstruation | Male-pattern baldness |
| Increased muscle mass | Enlargement of clitoris |
| Increased perspiration | Growth spurt/closure of growth plates |
| Acne | Breast atrophy |
| Increased red blood cell count |
|
Safety
Hormone therapy for transgender individuals has been shown in medical literature to be generally safe, when supervised by a qualified medical professional. There are potential risks with hormone treatment that will be monitored through screenings and lab tests such as blood count (hemoglobin), kidney and liver function, blood sugar, potassium, and cholesterol. Taking more medication than directed may lead to health problems such as increased risk of cancer, heart attack from thickening of the blood, blood clots, and elevated cholesterol.
Feminizing hormone therapy
The Standards of Care published by the World Professional Association for Transgender Health (WPATH) summarize many of the risks associated with feminizing hormone therapy (outlined below). For more in-depth information on the safety profile of estrogen-based feminizing hormone therapy visit the feminizing hormone therapy page.
| Likely Increased Risk | Possible Increased Risk | Inconclusive/No Increased Risk |
|---|---|---|
| Venous thromboembolic disease | Type 2 diabetes | Breast cancer |
| Cardiovascular disease | Hypertension | Prostate cancer |
| Hypertriglyceridemia | Hyperprolactinaemia |
|
| Gallstones | Osteoporosis |
|
| Hyperkalemia |
|
|
| Cerebrovascular disease |
|
|
| Polyuria (or dehydration) |
|
|
| Meningioma |
|
|
|
Masculinizing hormone therapy
The Standards of Care published by the World Professional Association for Transgender Health (WPATH) summarize many of the risks associated with masculinizing hormone therapy (outlined below). For more in-depth information on the safety profile of testosterone-based masculinizing hormone therapy visit the masculinizing hormone therapy page.
| Likely Increased Risk | Possible Increased Risk | Inconclusive/No Increased Risk |
|---|---|---|
| Polycythemia | Type 2 diabetes | Osteoporosis |
| Weight gain |
|
Breast cancer |
| Acne |
|
Ovarian cancer |
| Pattern hair loss |
|
Uterine cancer |
| Hypertension |
|
Cervical cancer |
| Sleep apnea |
|
|
| Decreased HDL cholesterol |
|
|
| Decreased LDL cholesterol |
|
|
| Cardiovascular disease |
|
|
| Hypertriglyceridemia |
|
|
Fertility consideration
GAHT may limit fertility potential. Should a transgender individual choose to undergo gender-affirming surgery, their fertility potential is lost completely. Before starting any treatment, individuals may consider fertility issues and fertility preservation. Options include semen cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation.
A study presented at ENDO 2019 (the Endocrine Society's conference) shows that even after one year of treatment with testosterone, a transgender man can preserve his fertility potential.
Fake products
Some online scammers have been targeting trans consumers with products that do not contain any hormones or contain ones that are opposite of what is advertised. This can happen when legislations outlaw or restrict access to treatments by legitimate medical professionals.
Treatment eligibility
Many providers use informed consent, whereby someone seeking hormone therapy can sign a statement of informed consent and begin treatment without much gatekeeping. For other providers, eligibility is determined using major diagnostic tools such as ICD-11 or the Diagnostic and Statistical Manual of Mental Disorders (DSM) to classify a patient with gender dysphoria. The Endocrine Society requires physicians that diagnose gender dysphoria and gender incongruence to be trained in psychiatric disorders with competency in ICD-11 and DSM-5. The healthcare provider should also obtain a thorough assessment of the patient's mental health and identify potential psychosocial factors that can affect therapy.
WPATH Standards of Care
The WPATH Standards of Care, most recently published in 2022, outlines a series of guidelines which should be met before a patient should be allowed gender-affirming hormone therapy:
- Gender incongruence is marked and sustained
- Patient meets diagnostic criteria for gender incongruence prior to gender-affirming hormone treatment in regions where a diagnosis is necessary to access health care
- Patient has capacity to consent to hormone therapy treatment
- Other possible causes of apparent gender incongruence have been identified and excluded
- Mental health and physical conditions that could negatively impact the outcome of treatment have been assessed
- Understands the effect of gender-affirming hormone treatment on reproduction and they have explored reproductive options
The WPATH standards of care distinguish between gender-affirming hormone therapy, and hormone replacement therapy, with the latter referring to the replacement of endogenous hormones after a gonadectomy to prevent cardiovascular and musculoskeletal issues.
Readiness
Some organizations – but fewer than in the past – require that patients spend a certain period of time living in their desired gender role before starting hormone therapy. This period is sometimes called real-life experience (RLE).
In Sweden, for instance, patients seeking to access gender affirming healthcare must first undergo extended evaluations with psychiatric professionals, during which they must - without any form of medical transition - successfully live for one full year as their desired gender in all professional, social, and personal matters. Gender clinics are recommended to provide patients with wigs and breast prostheses for the endeavor. The evaluation additionally involves, if possible, meetings with family members and/or other individuals close to the patient. Patients may be denied care for any number of "psychosocial dimensions", including their choice of job or their marital status.
Transgender and gender non-conforming activists, such as Kate Bornstein, have asserted that RLE is psychologically harmful and is a form of "gatekeeping", effectively barring individuals from transitioning for as long as possible, if not permanently.
In September 2022, the World Professional Association for Transgender Health (WPATH) Standards of Care for the Health of Transgender and Gender Diverse People (SOC) Version 8 were released and removed the requirement of RLE for all gender-affirming treatments, including gender-affirming surgery.
Accessibility
Some transgender people choose to self-administer hormone replacement medications, often because doctors have too little experience in this area, or because no doctor is available. Others self-administer because their doctor will not prescribe hormones without an approval letter from a psychotherapist. Many therapists require extended periods of continuous psychotherapy and/or real-life experience before they will write such a letter. Because many individuals must pay for evaluation and care out-of-pocket, costs can be prohibitive.
Access to medication can be poor even where health care is provided free. In a patient survey conducted by the United Kingdom's National Health Service in 2008, 5% of respondents acknowledged resorting to self-medication, and 46% were dissatisfied with the amount of time it took to receive hormone therapy. The report concluded in part: "The NHS must provide a service that is easy to access so that vulnerable patients do not feel forced to turn to DIY remedies such as buying drugs online with all the risks that entails. Patients must be able to access professional help and advice so that they can make informed decisions about their care, whether they wish to take the NHS or private route without putting their health and indeed their lives in danger." Self-administration of cross-gender hormones without medical supervision may have untoward health effects and risks.
A number of private companies have attempted to increase accessibility for hormone replacement medications and help transgender people navigate the complexities of access to treatment.
