From Wikipedia, the free encyclopedia
Example of an approximately 40,000 probe spotted oligo
microarray with enlarged inset to show detail.
Biomedical engineering (
BME) is the application of
engineering principles and design concepts to medicine and biology for
healthcare purposes (e.g. diagnostic or therapeutic). This field seeks
to close the gap between
engineering and
medicine,
combining the design and problem solving skills of engineering with
medical biological sciences to advance health care treatment, including
diagnosis,
monitoring, and
therapy.
[1]
Biomedical engineering has only recently emerged as its own study, as
compared to many other engineering fields. Such an evolution is common
as a new field transitions from being an
interdisciplinary
specialization among already-established fields, to being considered a
field in itself. Much of the work in biomedical engineering consists of
research and development, spanning a broad array of subfields (see below). Prominent biomedical engineering applications include the development of
biocompatible prostheses, various diagnostic and therapeutic
medical devices ranging from clinical equipment to micro-implants, common imaging equipment such as
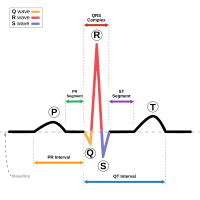
MRIs and
EKG/ECGs,
regenerative tissue growth, pharmaceutical
drugs and therapeutic biologicals.
Bioinformatics
Bioinformatics is an interdisciplinary field that develops
methods and software tools for understanding biological data. As an
interdisciplinary field of science, bioinformatics combines computer
science, statistics, mathematics, and engineering to analyze and
interpret biological data.
Bioinformatics is both an umbrella term for the body of biological
studies that use computer programming as part of their methodology, as
well as a reference to specific analysis "pipelines" that are repeatedly
used, particularly in the field of genomics. Common uses of
bioinformatics include the identification of candidate genes and
nucleotides (SNPs). Often, such identification is made with the aim of
better understanding the genetic basis of disease, unique adaptations,
desirable properties (esp. in agricultural species), or differences
between populations. In a less formal way, bioinformatics also tries to
understand the organisational principles within nucleic acid and protein
sequences.
Biomechanics
Biomechanics is the study of the structure and function of the
mechanical aspects of biological systems, at any level from whole
organisms to
organs,
cells and
cell organelles,
[2] using the methods of
mechanics.
[3]
Biomaterial
A
biomaterial is any matter, surface, or construct that interacts with living systems. As a science,
biomaterials is about fifty years old. The study of biomaterials is called
biomaterials science or biomaterials engineering.
It has experienced steady and strong growth over its history, with many
companies investing large amounts of money into the development of new
products. Biomaterials science encompasses elements of medicine,
biology, chemistry, tissue engineering and materials science.
Biomedical optics
Biomedical
optics refers to the interaction of biological tissue and light, and
how this can be exploited for sensing, imaging, and treatment.
[4]
Tissue engineering
Tissue engineering, like genetic engineering (see below), is a major segment of
biotechnology – which overlaps significantly with BME.
One of the goals of tissue engineering is to create artificial organs
(via biological material) for patients that need organ transplants.
Biomedical engineers are currently researching methods of creating such
organs. Researchers have grown solid
jawbones[5] and
tracheas[6] from human stem cells towards this end. Several
artificial urinary bladders have been grown in laboratories and transplanted successfully into human patients.
[7]
Bioartificial organs, which use both synthetic and biological
component, are also a focus area in research, such as with hepatic
assist devices that use liver cells within an artificial bioreactor
construct.
[8]
Micromass cultures of C3H-10T1/2 cells at varied oxygen tensions stained with
Alcian blue.
Genetic engineering
Genetic engineering, recombinant DNA technology, genetic
modification/manipulation (GM) and gene splicing are terms that apply to
the direct manipulation of an organism's genes. Unlike traditional
breeding, an indirect method of genetic manipulation, genetic
engineering utilizes modern tools such as molecular cloning and
transformation to directly alter the structure and characteristics of
target genes. Genetic engineering techniques have found success in
numerous applications. Some examples include the improvement of crop
technology (
not a medical application, but see
biological systems engineering),
the manufacture of synthetic human insulin through the use of modified
bacteria, the manufacture of erythropoietin in hamster ovary cells, and
the production of new types of experimental mice such as the oncomouse
(cancer mouse) for research.
Neural engineering
Neural engineering
(also known as neuroengineering) is a discipline that uses engineering
techniques to understand, repair, replace, or enhance neural systems.
Neural engineers are uniquely qualified to solve design problems at the
interface of living neural tissue and non-living constructs.
Pharmaceutical engineering
Pharmaceutical engineering
is an interdisciplinary science that includes drug engineering, novel
drug delivery and targeting, pharmaceutical technology, unit operations
of
Chemical Engineering, and Pharmaceutical Analysis. It may be deemed as a part of
pharmacy due to its focus on the use of technology on chemical agents in providing better medicinal treatment. The
ISPE is an international body that certifies this now rapidly emerging interdisciplinary science.
Medical devices
This is an
extremely broad category—essentially covering all
health care products that do not achieve their intended results through
predominantly chemical (e.g., pharmaceuticals) or biological (e.g.,
vaccines) means, and do not involve metabolism.
A medical device is intended for use in:
- the diagnosis of disease or other conditions, or
- in the cure, mitigation, treatment, or prevention of disease.
Some examples include
pacemakers,
infusion pumps, the
heart-lung machine,
dialysis machines,
artificial organs,
implants,
artificial limbs,
corrective lenses,
cochlear implants,
ocular prosthetics,
facial prosthetics, somato prosthetics, and
dental implants.
Stereolithography is a practical example of
medical modeling
being used to create physical objects. Beyond modeling organs and the
human body, emerging engineering techniques are also currently used in
the research and development of new devices for innovative therapies,
[9] treatments,
[10] patient monitoring,
[11] of complex diseases.
Medical devices are regulated and classified (in the US) as follows (see also
Regulation):
- Class I devices present minimal potential for harm to the user and
are often simpler in design than Class II or Class III devices. Devices
in this category include tongue depressors, bedpans, elastic bandages,
examination gloves, and hand-held surgical instruments and other similar
types of common equipment.
- Class II devices are subject to special controls in addition to the
general controls of Class I devices. Special controls may include
special labeling requirements, mandatory performance standards, and postmarket surveillance.
Devices in this class are typically non-invasive and include X-ray
machines, PACS, powered wheelchairs, infusion pumps, and surgical
drapes.
- Class III devices generally require premarket approval (PMA) or
premarket notification (510k), a scientific review to ensure the
device's safety and effectiveness, in addition to the general controls
of Class I. Examples include replacement heart valves, hip and knee
joint implants, silicone gel-filled breast implants, implanted
cerebellar stimulators, implantable pacemaker pulse generators and
endosseous (intra-bone) implants.
Medical imaging
Medical/biomedical imaging is a major segment of
medical devices.
This area deals with enabling clinicians to directly or indirectly
"view" things not visible in plain sight (such as due to their size,
and/or location). This can involve utilizing ultrasound, magnetism, UV,
radiology, and other means.
Imaging technologies are often essential to medical diagnosis, and
are typically the most complex equipment found in a hospital including:
fluoroscopy,
magnetic resonance imaging (MRI),
nuclear medicine,
positron emission tomography (PET),
PET-CT scans, projection radiography such as
X-rays and
CT scans,
tomography,
ultrasound,
optical microscopy, and
electron microscopy.
Implants
An
implant is a kind of medical device made to replace and act as a missing
biological structure (as compared with a transplant, which indicates
transplanted biomedical tissue). The surface of implants that contact
the body might be made of a biomedical material such as titanium,
silicone or apatite depending on what is the most functional. In some
cases, implants contain electronics, e.g. artificial pacemakers and
cochlear implants. Some implants are bioactive, such as subcutaneous
drug delivery devices in the form of implantable pills or
drug-eluting stents.
Bionics
Artificial body part replacements are one of the many applications of
bionics. Concerned with the intricate and thorough study of the
properties and function of human body systems, bionics may be applied to
solve some engineering problems. Careful study of the different
functions and processes of the eyes, ears, and other organs paved the
way for improved cameras, television, radio transmitters and receivers,
and many other useful tools. These developments have indeed made our
lives better, but the best contribution that bionics has made is in the
field of biomedical engineering (the building of useful replacements for
various parts of the human body). Modern hospitals now have available
spare parts to replace body parts badly damaged by injury or disease
[Citation Needed]. Biomedical engineers work hand in hand with doctors
to build these artificial body parts.
Clinical engineering
Clinical engineering is the branch of biomedical engineering dealing with the actual implementation of
medical equipment and technologies in hospitals or other clinical settings. Major roles of clinical engineers include training and supervising
biomedical equipment technicians (BMETs),
selecting technological products/services and logistically managing
their implementation, working with governmental regulators on
inspections/audits, and serving as technological consultants for other
hospital staff (e.g. physicians, administrators, I.T., etc.). Clinical
engineers also advise and collaborate with medical device producers
regarding prospective design improvements based on clinical experiences,
as well as monitor the progression of the state of the art so as to
redirect procurement patterns accordingly.
Their inherent focus on
practical implementation of technology has tended to keep them oriented more towards
incremental-level
redesigns and reconfigurations, as opposed to revolutionary research
& development or ideas that would be many years from clinical
adoption; however, there is a growing effort to expand this time-horizon
over which clinical engineers can influence the trajectory of
biomedical innovation. In their various roles, they form a "bridge"
between the primary designers and the end-users, by combining the
perspectives of being both 1) close to the point-of-use, while 2)
trained in product and process engineering. Clinical engineering
departments will sometimes hire not just biomedical engineers, but also
industrial/systems engineers to help address operations
research/optimization, human factors, cost analysis, etc. Also see
safety engineering for a discussion of the procedures used to design safe systems.
Rehabilitation engineering
Rehabilitation engineering is the systematic application of
engineering sciences to design, develop, adapt, test, evaluate, apply,
and distribute technological solutions to problems confronted by
individuals with disabilities. Functional areas addressed through
rehabilitation engineering may include mobility, communications,
hearing, vision, and cognition, and activities associated with
employment, independent living, education, and integration into the
community.
[1]
While some rehabilitation engineers have master's degrees in
rehabilitation engineering, usually a subspecialty of Biomedical
engineering, most rehabilitation engineers have undergraduate or
graduate degrees in biomedical engineering, mechanical engineering, or
electrical engineering. A Portuguese university provides an
undergraduate degree and a master's degree in Rehabilitation Engineering
and Accessibility.
[5][7]
Qualification to become a Rehab' Engineer in the UK is possible via a
University BSc Honours Degree course such as Health Design &
Technology Institute, Coventry University.
[8]
The rehabilitation process for people with disabilities often entails
the design of assistive devices such as Walking aids intended to
promote inclusion of their users into the mainstream of society,
commerce, and recreation.
Regulatory issues
Regulatory issues have been constantly increased in the last decades
to respond to the many incidents caused by devices to patients. For
example, from 2008 to 2011, in US, there were 119 FDA recalls of medical
devices classified as class I. According to U.S. Food and Drug
Administration (FDA),
Class I recall
is associated to "a situation in which there is a reasonable
probability that the use of, or exposure to, a product will cause
serious adverse health consequences or death"
[12]
Regardless of the country-specific legislation, the main regulatory objectives coincide worldwide.
[13] For example, in the medical device regulations, a product must be: 1) safe
and 2) effective and 3) for all the manufactured devices
A product is safe if patients, users and third parties do not run
unacceptable risks of physical hazards (death, injuries, …) in its
intended use. Protective measures have to be introduced on the devices
to reduce residual risks at acceptable level if compared with the
benefit derived from the use of it.
A product is effective if it performs as specified by the
manufacturer in the intended use. Effectiveness is achieved through
clinical evaluation, compliance to performance standards or
demonstrations of substantial equivalence with an already marketed
device.
The previous features have to be ensured for all the manufactured
items of the medical device. This requires that a quality system shall
be in place for all the relevant entities and processes that may impact
safety and effectiveness over the whole medical device lifecycle.
The medical device engineering area is among the most heavily
regulated fields of engineering, and practicing biomedical engineers
must routinely consult and cooperate with regulatory law attorneys and
other experts. The Food and Drug Administration (FDA) is the principal
healthcare regulatory authority in the United States, having
jurisdiction over medical
devices, drugs, biologics, and combination
products. The paramount objectives driving policy decisions by the FDA
are safety and effectiveness of healthcare products that have to be
assured through a quality system in place as specified under
21 CFR 829 regulation.
In addition, because biomedical engineers often develop devices and
technologies for "consumer" use, such as physical therapy devices (which
are also "medical" devices), these may also be governed in some
respects by the
Consumer Product Safety Commission.
The greatest hurdles tend to be 510K "clearance" (typically for Class 2
devices) or pre-market "approval" (typically for drugs and class 3
devices).
In the European context, safety effectiveness and quality is ensured
through the "Conformity Assessment" that is defined as "the method by
which a manufacturer demonstrates that its device complies with the
requirements of the European
Medical Device Directive".
The directive specifies different procedures according to the class of
the device ranging from the simple Declaration of Conformity (Annex VII)
for Class I devices to EC verification (Annex IV), Production quality
assurance (Annex V), Product quality assurance (Annex VI) and Full
quality assurance (Annex II). The Medical Device Directive specifies
detailed procedures for Certification. In general terms, these
procedures include tests and verifications that are to be contained in
specific deliveries such as the risk management file, the technical file
and the quality system deliveries. The risk management file is the
first deliverable that conditions the following design and manufacturing
steps. Risk management stage shall drive the product so that product
risks are reduced at an acceptable level with respect to the benefits
expected for the patients for the use of the device. The
technical file
contains all the documentation data and records supporting medical
device certification. FDA technical file has similar content although
organized in different structure. The Quality System deliverables
usually includes procedures that ensure quality throughout all product
life cycle. The same standard (ISO EN 13485) is usually applied for
quality management systems in US and worldwide.
Implants, such as
artificial hip joints, are generally extensively regulated due to the invasive nature of such devices.
In the European Union, there are certifying entities named "
Notified Bodies",
accredited by European Member States. The Notified Bodies must ensure
the effectiveness of the certification process for all medical devices
apart from the class I devices where a declaration of conformity
produced by the manufacturer is sufficient for marketing. Once a product
has passed all the steps required by the Medical Device Directive, the
device is entitled to bear a
CE marking,
indicating that the device is believed to be safe and effective when
used as intended, and, therefore, it can be marketed within the European
Union area.
The different regulatory arrangements sometimes result in particular
technologies being developed first for either the U.S. or in Europe
depending on the more favorable form of regulation. While nations often
strive for substantive harmony to facilitate cross-national
distribution, philosophical differences about the
optimal extent
of regulation can be a hindrance; more restrictive regulations seem
appealing on an intuitive level, but critics decry the tradeoff cost in
terms of slowing access to life-saving developments.
RoHS II
Directive
2011/65/EU, better known as RoHS 2 is a recast of legislation
originally introduced in 2002. The original EU legislation "Restrictions
of Certain Hazardous Substances in Electrical and Electronics Devices"
(RoHS Directive 2002/95/EC) was replaced and superseded by 2011/65/EU
published in July 2011 and commonly known as RoHS 2.
RoHS
seeks to limit the dangerous substances in circulation in electronics
products, in particular toxins and heavy metals, which are subsequently
released into the environment when such devices are recycled.
The scope of RoHS 2 is widened to include products previously
excluded, such as medical devices and industrial equipment. In addition,
manufacturers are now obliged to provide conformity risk assessments
and test reports – or explain why they are lacking. For the first time,
not only manufacturers, but also importers and distributors share a
responsibility to ensure Electrical and Electronic Equipment within the
scope of RoHS comply with the hazardous substances limits and have a CE
mark on their products.
IEC 60601
The new International Standard
IEC 60601
for home healthcare electro-medical devices defining the requirements
for devices used in the home healthcare environment. IEC 60601-1-11
(2010) must now be incorporated into the design and verification of a
wide range of home use and point of care medical devices along with
other applicable standards in the IEC 60601 3rd edition series.
The mandatory date for implementation of the EN European version of
the standard is June 1, 2013. The US FDA requires the use of the
standard on June 30, 2013, while Health Canada recently extended the
required date from June 2012 to April 2013. The North American agencies
will only require these standards for new device submissions, while the
EU will take the more severe approach of requiring all applicable
devices being placed on the market to consider the home healthcare
standard.
AS/NZS 3551:2012
AS/ANS 3551:2012
is the Australian and New Zealand standards for the management of
medical devices. The standard specifies the procedures required to
maintain a wide range of medical assets in a clinical setting (e.g.
Hospital).
[14] The standards are based on the IEC 606101 standards.
The standard covers a wide range of medical equipment management
elements including, procurement, acceptance testing, maintenance
(electrical safety and preventative maintenance testing) and
decommissioning.
Training and certification
Education
Biomedical
engineers require considerable knowledge of both engineering and
biology, and typically have a Bachelor's (B.Tech, B.S) or Master's
(M.S., M.Tech, M.S.E., or M.Eng.) or a Doctoral (Ph.D.) degree in BME
(Biomedical Engineering) or another branch of engineering with
considerable potential for BME overlap. As interest in BME increases,
many engineering colleges now have a Biomedical Engineering Department
or Program, with offerings ranging from the undergraduate (B.Tech, B.S.,
B.Eng or B.S.E.) to doctoral levels. Biomedical engineering has only
recently been emerging as
its own discipline rather than a
cross-disciplinary hybrid specialization of other disciplines; and BME
programs at all levels are becoming more widespread, including the
Bachelor of Science in Biomedical Engineering which actually includes so much biological science content that many students use it as a "
pre-med" major in preparation for
medical school. The number of biomedical engineers is expected to rise as both a cause and effect of improvements in medical technology.
[15]
In the U.S., an increasing number of
undergraduate programs are also becoming recognized by
ABET as accredited bioengineering/biomedical engineering programs. Over 65 programs are currently accredited by ABET.
[16][17]
In Canada and Australia, accredited graduate programs in Biomedical Engineering are common, for example in Universities such as
McMaster University, and the first Canadian
undergraduate BME program at
Ryerson University offering a four-year B.Eng program.
[18][19][20][21] The Polytechnique in Montreal is also offering a bachelors's degree in biomedical engineering.
As with many degrees, the reputation and ranking of a program may
factor into the desirability of a degree holder for either employment or
graduate admission. The reputation of many undergraduate degrees are
also linked to the institution's graduate or research programs, which
have some tangible factors for rating, such as research funding and
volume, publications and citations. With BME specifically, the ranking
of a university's hospital and medical school can also be a significant
factor in the perceived prestige of its BME department/program.
Graduate education
is a particularly important aspect in BME. While many engineering
fields (such as mechanical or electrical engineering) do not need
graduate-level training to obtain an entry-level job in their field, the
majority of BME positions do prefer or even require them.
[22] Since most BME-related professions involve scientific research, such as in
pharmaceutical and
medical device
development, graduate education is almost a requirement (as
undergraduate degrees typically do not involve sufficient research
training and experience). This can be either a Masters or Doctoral level
degree; while in certain specialties a Ph.D. is notably more common
than in others, it is hardly ever the majority (except in academia). In
fact, the perceived need for some kind of graduate credential is so
strong that some undergraduate BME programs will actively discourage
students from majoring in BME without an expressed intention to also
obtain a master's degree or apply to medical school afterwards.
Graduate programs in BME, like in other scientific fields, are highly
varied, and particular programs may emphasize certain aspects within
the field. They may also feature extensive collaborative efforts with
programs in other fields (such as the University's Medical School or
other engineering divisions), owing again to the interdisciplinary
nature of BME. M.S. and Ph.D. programs will typically require applicants
to have an undergraduate degree in BME, or
another engineering discipline (plus certain life science coursework), or
life science (plus certain engineering coursework).
Education in BME also varies greatly around the world. By virtue of
its extensive biotechnology sector, its numerous major universities, and
relatively few internal barriers, the U.S. has progressed a great deal
in its development of BME education and training opportunities. Europe,
which also has a large biotechnology sector and an impressive education
system, has encountered trouble in creating uniform standards as the
European community attempts to supplant some of the national
jurisdictional barriers that still exist. Recently, initiatives such as
BIOMEDEA have sprung up to develop BME-related education and
professional standards.
[23] Other countries, such as Australia, are recognizing and moving to correct deficiencies in their BME education.
[24]
Also, as high technology endeavors are usually marks of developed
nations, some areas of the world are prone to slower development in
education, including in BME.
Licensure/certification
As with other learned professions, each state has certain (fairly similar) requirements for becoming licensed as a registered
Professional Engineer
(PE), but, in US, in industry such a license is not required to be an
employee as an engineer in the majority of situations (due to an
exception known as the industrial exemption, which effectively applies
to the vast majority of American engineers). The US model has generally
been only to require the practicing engineers offering engineering
services that impact the public welfare, safety, safeguarding of life,
health, or property to be licensed, while engineers working in private
industry without a direct offering of engineering services to the public
or other businesses, education, and government need not be licensed.
This is notably not the case in many other countries, where a license is
as legally necessary to practice engineering as it is for law or
medicine.
Biomedical engineering is regulated in some countries, such as
Australia, but registration is typically only recommended and not
required.
[25]
In the UK, mechanical engineers working in the areas of Medical Engineering,
Bioengineering or Biomedical engineering can gain
Chartered Engineer status through the
Institution of Mechanical Engineers. The Institution also runs the Engineering in Medicine and Health Division.
[26]
The Institute of Physics and Engineering in Medicine (IPEM) has a panel
for the accreditation of MSc courses in Biomedical Engineering and
Chartered Engineering status can also be sought through IPEM.
The
Fundamentals of Engineering exam
– the first (and more general) of two licensure examinations for most
U.S. jurisdictions—does now cover biology (although technically not
BME). For the second exam, called the Principles and Practices, Part 2,
or the Professional Engineering exam, candidates may select a particular
engineering discipline's content to be tested on; there is currently
not an option for BME with this, meaning that any biomedical engineers
seeking a license must prepare to take this examination in another
category (which does not affect the actual license, since most
jurisdictions do not recognize discipline specialties anyway). However,
the Biomedical Engineering Society (BMES) is, as of 2009, exploring the
possibility of seeking to implement a BME-specific version of this exam
to facilitate biomedical engineers pursuing licensure.
Beyond governmental registration, certain private-sector
professional/industrial organizations also offer certifications with
varying degrees of prominence. One such example is the Certified
Clinical Engineer (CCE) certification for Clinical engineers.
Career prospects
In
2012 there were about 19,400 biomedical engineers employed in the US,
and the field was predicted to grow by 27% (much faster than average)
from 2012 to 2022.
[27] Biomedical engineering has the highest percentage of women engineers compared to other common engineering professions.
Notable figures
- Forrest Bird (deceased) – aviator and pioneer in the invention of mechanical ventilators
- Y.C. Fung – professor emeritus at the University of California, San Diego, considered by many to be the founder of modern biomechanics[28]
- Leslie Geddes (deceased) – professor emeritus at Purdue University, electrical engineer, inventor, and educator of over 2000 biomedical engineers, received a National Medal of Technology in 2006 from President George Bush[29]
for his more than 50 years of contributions that have spawned
innovations ranging from burn treatments to miniature defibrillators,
ligament repair to tiny blood pressure monitors for premature infants,
as well as a new method for performing cardiopulmonary resuscitation (CPR).
- Willem Johan Kolff (deceased) – pioneer of hemodialysis as well as in the field of artificial organs
- Robert Langer – Institute Professor at MIT, runs the largest BME laboratory in the world, pioneer in drug delivery and tissue engineering[30]
- John Macleod (deceased) – one of the co-discoverers of insulin at Case Western Reserve University.
- Alfred E. Mann – Physicist, entrepreneur and philanthropist. A pioneer in the field of Biomedical Engineering.[31]
- Nicholas A. Peppas – Chaired Professor in Engineering, University of Texas at Austin, pioneer in drug delivery, biomaterials, hydrogels and nanobiotechnology.
- Robert Plonsey – professor emeritus at Duke University, pioneer of electrophysiology[32]
- Robert M. Nerem – professor emeritus at Georgia Institute of Technology.
Pioneer in regenerative tissue, biomechanics, and author of over 300
published works. His works have been cited more than 20,000 times
cumulatively.
- Otto Schmitt (deceased) – biophysicist with significant contributions to BME, working with biomimetics
- Ascher Shapiro
(deceased) – Institute Professor at MIT, contributed to the development
of the BME field, medical devices (e.g. intra-aortic balloons)
- John G. Webster – professor emeritus at the University of Wisconsin–Madison, a pioneer in the field of instrumentation amplifiers for the recording of electrophysiological signals
- U.A. Whitaker (deceased) – provider of the Whitaker Foundation,
which supported research and education in BME by providing over $700
million to various universities, helping to create 30 BME programs and
helping finance the construction of 13 buildings[33]