From Wikipedia, the free encyclopedia
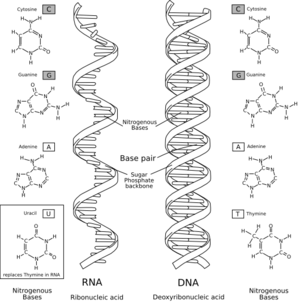
A comparison of RNA (
left) with DNA (
right), showing the helices and
nucleobases each employs
The
RNA world refers to the self-replicating
ribonucleic acid (RNA) molecules hypothesised to have been the precursors to all current life on Earth.
[1][2][3] The hypothesis that current
life on Earth descends from an RNA world is widely accepted,
[4][5] and is supported by several lines of evidence including how ephemeral RNA was protected on the early earth.
[6] Alternative chemical paths to life have been proposed,
[7] and RNA-based life may not have been the first life to exist.
[8][9]
The RNA world would have eventually been replaced by the
DNA-RNA-protein world of today, likely through an intermediate stage of
ribonucleoprotein enzymes such as the
ribosome and
ribozymes,
since it is argued that proteins large enough to self-fold and have
useful activities would only have come about after RNA was available to
catalyze
peptide ligation or
amino acid polymerization.
[9] DNA is thought to have taken over the role of data storage due to its increased stability,
[10] while proteins, through a greater variety of
monomers (amino acids), replaced RNA's role in specialized
biocatalysis.
The RNA world hypothesis is supported by many independent lines of
evidence, such as the observations that RNA is central to the
translation process and that small RNAs can catalyze all of the chemical
group and information transfers required for life.
[9][11]
The structure of the ribosome has been called the "smoking gun," as it
showed that the ribosome is a ribozyme, with a central core of RNA and
no amino acid side chains within 18 angstroms of the
active site where peptide bond formation is catalyzed.
[8] Many of the most critical components of cells (those that
evolve the slowest) are composed mostly or entirely of RNA. Also, many critical
cofactors (
ATP,
Acetyl-CoA,
NADH, etc.) are either
nucleotides
or substances clearly related to them. This would mean that the RNA and
nucleotide cofactors in modern cells could be an evolutionary remnant
of an RNA-based enzymatic system that preceded the protein-based one
seen in all extant life.
History
One of the challenges in studying
abiogenesis
is that the system of reproduction and metabolism utilized by all
extant life involves three distinct types of interdependent
macromolecules (
DNA,
RNA, and
protein).
This suggests that life could not have arisen in its current form, and
mechanisms have then been sought whereby the current system might have
arisen from a simpler precursor system. The concept of RNA as a
primordial molecule
[9] can be found in papers by
Francis Crick[12] and
Leslie Orgel,
[13] as well as in
Carl Woese's 1967 book
The Genetic Code.
[14] In 1962 the molecular biologist
Alexander Rich, of the
Massachusetts Institute of Technology, had posited much the same idea in an article he contributed to a volume issued in honor of Nobel-laureate physiologist
Albert Szent-Györgyi.
[15] Hans Kuhn
in 1972 laid out a possible process by which the modern genetic system
might have arisen from a nucleotide-based precursor, and this led Harold
White in 1976 to observe that many of the cofactors essential for
enzymatic function are either nucleotides or could have been derived
from nucleotides. He proposed that these nucleotide cofactors represent
"fossils of nucleic acid enzymes".
[16] The phrase "RNA World" was first used by Nobel laureate
Walter Gilbert
in 1986, in a commentary on how recent observations of the catalytic
properties of various forms of RNA fit with this hypothesis.
[17]
Properties of RNA
The
properties of RNA make the idea of the RNA world hypothesis
conceptually plausible, though its general acceptance as an explanation
for the origin of life requires further evidence.
[15]
RNA is known to form efficient catalysts and its similarity to DNA
makes clear its ability to store information. Opinions differ, however,
as to whether RNA constituted the first autonomous self-replicating
system or was a derivative of a still-earlier system.
[9] One version of the hypothesis is that a different type of
nucleic acid, termed
pre-RNA,
was the first one to emerge as a self-reproducing molecule, to be
replaced by RNA only later. On the other hand, the recent finding that
activated
pyrimidine ribonucleotides can be synthesized under plausible
prebiotic conditions
[18] means that it is premature to dismiss the RNA-first scenarios.
[9] Suggestions for 'simple'
pre-RNA nucleic acids have included
peptide nucleic acid (PNA),
threose nucleic acid (TNA) or
glycol nucleic acid (GNA).
[19][20]
Despite their structural simplicity and possession of properties
comparable with RNA, the chemically plausible generation of "simpler"
nucleic acids under prebiotic conditions has yet to be demonstrated.
[21]
RNA as an enzyme
RNA enzymes, or ribozymes, are found in today's DNA-based life and could be examples of
living fossils. Ribozymes play vital roles, such as those in the
ribosome, which is vital for protein synthesis. Many other ribozyme functions exist; for example, the
hammerhead ribozyme performs self-cleavage
[22] and an
RNA polymerase ribozyme can synthesize a short RNA strand from a primed RNA template.
[23]
Among the enzymatic properties important for the beginning of life are:
- Self-replication. The ability to self-replicate,
or synthesize other RNA molecules; relatively short RNA molecules that
can synthesize others have been artificially produced in the lab. The
shortest was 165-bases long, though it has been estimated that only part
of the molecule was crucial for this function. One version, 189-bases
long, had an error rate of just 1.1% per nucleotide when synthesizing an
11 nucleotide long RNA strand from primed template strands.[24]
This 189 base pair ribozyme could polymerize a template of at most 14
nucleotides in length, which is too short for self replication, but a
potential lead for further investigation. The longest primer extension performed by a ribozyme polymerase was 20 bases.[25]
In 2016, researchers reported the use of in vitro evolution to improve
dramatically the activity and generality of an RNA polymerase ribozyme
by selecting variants that can synthesize functional RNA molecules from
an RNA template. Each RNA polymerase ribozyme was engineered to remain
linked to its new, synthesized RNA strand, this allowed the team to
isolate successful polymerases. The isolated RNA polymerases were again
used for another round of evolution. After several rounds of evolution,
they obtained one RNA polymerase ribozyme called 24-3 that was able to
copy almost any other RNA, from small catalysts to long RNA based
enzymes. Particular RNAs were amplified up to 10,000 times, a first RNA
version of the polymerase chain reaction (PCR). The RNA polymerase is
not yet able to make copies of itself.[26]
- Catalysis. The ability to catalyze
simple chemical reactions—which would enhance creation of molecules
that are building blocks of RNA molecules (i.e., a strand of RNA which
would make creating more strands of RNA easier). Relatively short RNA
molecules with such abilities have been artificially formed in the lab.[27][28]
A recent study showed that almost any nucleic acid can evolve into a
catalytic sequence under appropriate selection. For instance, an
arbitrarily chosen 50-nucleotide DNA fragment encoding for the Bos taurus (cattle) albumin
mRNA was subjected to test-tube evolution to derive a catalytic DNA
(DNAzyme) with RNA-cleavage activity. After only a few weeks, a DNAzyme
with significant catalytic activity had evolved.[29]
In general, DNA is much more chemically inert than RNA and hence much
more resistant to obtaining catalytic properties. If in vitro evolution
works for DNA it will happen much more easily with RNA.
- Amino acid-RNA ligation. The ability to conjugate an amino acid to the 3'-end of an RNA in order to use its chemical groups or provide a long-branched aliphatic side-chain.[30]
- Peptide bond formation. The ability to catalyse the formation of peptide bonds between amino acids to produce short peptides or longer proteins. This is done in modern cells by ribosomes, a complex of several RNA molecules known as rRNA
together with many proteins. The rRNA molecules are thought responsible
for its enzymatic activity, as no amino acid molecules lie within 18Å of the enzyme's active site,[15] and, when the majority of the amino acids in the ribosome were stringently removed, the resulting ribosome retained its full peptidyl transferase activity, fully able to catalyze the formation of peptide bonds between amino acids.[31] A much shorter RNA molecule has been synthesized in the laboratory with the ability to form peptide bonds, and it has been suggested that rRNA has evolved from a similar molecule.[32]
It has also been suggested that amino acids may have initially been
involved with RNA molecules as cofactors enhancing or diversifying their
enzymatic capabilities, before evolving to more complex peptides.
Similarly, tRNA is suggested to have evolved from RNA molecules that began to catalyze amino acid transfer.[33]
RNA in information storage
RNA
is a very similar molecule to DNA, and only has two chemical
differences. The overall structure of RNA and DNA are immensely
similar—one strand of DNA and one of RNA can bind to form a double
helical structure. This makes the storage of information in RNA possible
in a very similar way to the storage of information in DNA. However RNA
is less stable.
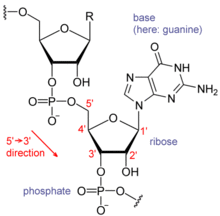
The major difference between RNA and DNA is the presence of a
hydroxyl group at the 2'-position.
Comparison of DNA and RNA structure
The major difference between RNA and DNA is the presence of a
hydroxyl group at the 2'-position of the
ribose sugar in RNA (illustration, right).
[15]
This group makes the molecule less stable because when not constrained
in a double helix, the 2' hydroxyl can chemically attack the adjacent
phosphodiester bond to cleave the phosphodiester backbone. The hydroxyl group also forces the ribose into the C3'-
endo sugar conformation unlike the C2'-
endo conformation of the
deoxyribose sugar in DNA. This forces an RNA double helix to change from a
B-DNA structure to one more closely resembling
A-DNA.
RNA also uses a different set of bases than DNA—
adenine,
guanine,
cytosine and
uracil, instead of adenine, guanine, cytosine and
thymine. Chemically, uracil is similar to thymine, differing only by a
methyl group, and its production requires less energy.
[34]
In terms of base pairing, this has no effect. Adenine readily binds
uracil or thymine. Uracil is, however, one product of damage to cytosine
that makes RNA particularly susceptible to mutations that can replace a
GC base pair with a
GU (
wobble) or
AU base pair.
RNA is thought to have preceded DNA, because of their ordering in the
biosynthetic pathways. The deoxyribonucleotides used to make DNA are
made from ribonucleotides, the building blocks of RNA, by removing the
2'-hydroxyl group. As a consequence a cell must have the ability to make
RNA before it can make DNA.
Limitations of information storage in RNA
The chemical properties of RNA make large RNA
molecules inherently fragile, and they can easily be broken down into their constituent nucleotides through
hydrolysis.
[35][36]
These limitations do not make use of RNA as an information storage
system impossible, simply energy intensive (to repair or replace damaged
RNA molecules) and prone to mutation. While this makes it unsuitable
for current 'DNA optimised' life, it may have been acceptable for more
primitive life.
RNA as a regulator
Riboswitches have been found to act as regulators of gene expression, particularly in bacteria, but also in plants and
archaea. Riboswitches alter their
secondary structure in response to the binding of a
metabolite. This change in structure can result in the formation or disruption of a
terminator, truncating or permitting transcription respectively.
[37] Alternatively, riboswitches may bind or occlude the
Shine-Dalgarno sequence, affecting translation.
[38] It has been suggested that these originated in an RNA-based world.
[39] In addition,
RNA thermometers regulate gene expression in response to temperature changes.
[40]
Support and difficulties
The RNA world hypothesis is supported by RNA's ability to store, transmit, and duplicate
genetic information, as
DNA does. RNA can act as a ribozyme, a special type of
enzyme.
Because it can perform the tasks of both DNA and enzymes, RNA is
believed to have once been capable of supporting independent life forms.
[15] Some
viruses use RNA as their genetic material, rather than DNA.
[41] Further, while
nucleotides were not found in experiments based on
Miller-Urey experiment, their formation in prebiotically plausible conditions has now been reported, as noted above;
[18] the
purine base known as adenine is merely a
pentamer of
hydrogen cyanide. Experiments with basic ribozymes, like
Bacteriophage Qβ
RNA, have shown that simple self-replicating RNA structures can
withstand even strong selective pressures (e.g., opposite-chirality
chain terminators).
[42]
Since there were no known chemical pathways for the abiogenic synthesis of nucleotides from
pyrimidine nucleobases cytosine and uracil under prebiotic conditions, it is thought by some that nucleic acids did not contain these
nucleobases seen in life's nucleic acids.
[43]
The nucleoside cytosine has a half-life in isolation of 19 days at
100 °C (212 °F) and 17,000 years in freezing water, which some argue is
too short on the
geologic time scale for accumulation.
[44] Others have questioned whether
ribose and other backbone sugars could be stable enough to find in the original genetic material,
[45] and have raised the issue that all ribose molecules would have had to be the same
enantiomer, as any nucleotide of the wrong
chirality acts as a chain
terminator.
[46]
Pyrimidine ribonucleosides and their respective nucleotides have been
prebiotically synthesised by a sequence of reactions that by-pass free
sugars and assemble in a stepwise fashion by including nitrogenous and
oxygenous chemistries. In a series of publications,
John Sutherland and his team at the School of Chemistry,
University of Manchester, have demonstrated high yielding routes to
cytidine and
uridine ribonucleotides built from small 2 and 3 carbon fragments such as
glycolaldehyde,
glyceraldehyde or glyceraldehyde-3-phosphate,
cyanamide and
cyanoacetylene. One of the steps in this sequence allows the isolation of
enantiopure
ribose aminooxazoline if the enantiomeric excess of glyceraldehyde is
60% or greater, of possible interest towards biological homochirality.
[47]
This can be viewed as a prebiotic purification step, where the said
compound spontaneously crystallised out from a mixture of the other
pentose
aminooxazolines. Aminooxazolines can react with cyanoacetylene in a mild and highly
efficient manner, controlled by inorganic phosphate, to give the
cytidine ribonucleotides. Photoanomerization with
UV light
allows for inversion about the 1' anomeric centre to give the correct
beta stereochemistry; one problem with this chemistry is the selective
phosphorylation of alpha-cytidine at the 2' position.
[48]
However, in 2009 they showed that the same simple building blocks allow
access, via phosphate controlled nucleobase elaboration, to
2',3'-cyclic pyrimidine nucleotides directly, which are known to be able
to polymerise into RNA.
[18] Organic chemist Donna Blackmond described this finding as "strong evidence" in favour of the RNA world.
[49]
However, John Sutherland said that while his team's work suggests that
nucleic acids played an early and central role in the origin of life, it
did not necessarily support the RNA world hypothesis in the strict
sense, which he described as a "restrictive, hypothetical arrangement".
[50]
The Sutherland group's 2009 paper also highlighted the possibility
for the photo-sanitization of the pyrimidine-2',3'-cyclic phosphates.
[18]
A potential weakness of these routes is the generation of
enantioenriched glyceraldehyde, or its 3-phosphate derivative
(glyceraldehyde prefers to exist as its keto
tautomer dihydroxyacetone).
[citation needed]
On August 8, 2011, a report, based on
NASA studies with
meteorites found on
Earth, was published suggesting building blocks of RNA (adenine, guanine and related
organic molecules) may have been formed extraterrestrially in
outer space.
[51][52][53] On August 29, 2012, astronomers at
Copenhagen University reported the detection of a specific sugar molecule,
glycolaldehyde, in a distant star system. The molecule was found around the
protostellar binary
IRAS 16293-2422, which is located 400 light years from Earth.
[54][55]
Because glycolaldehyde is needed to form RNA, this finding suggests
that complex organic molecules may form in stellar systems prior to the
formation of planets, eventually arriving on young planets early in
their formation.
[56]
"Molecular biologist's dream"
"Molecular biologist's dream" is a phrase coined by
Gerald Joyce and
Leslie Orgel to refer to the problem of emergence of
self-replicating RNA molecules, as any movement towards an RNA world on a properly modeled prebiotic
early Earth would have been continuously suppressed by destructive reactions.
[57] It was noted that many of the steps needed for the
nucleotides formation do not proceed efficiently in
prebiotic conditions.
[58] Joyce and Orgel specifically referred the molecular biologist's dream to "a magic
catalyst" that could "convert the activated nucleotides to a random ensemble of
polynucleotide sequences, a subset of which had the ability to replicate".
[57]
Joyce and Orgel further argued that nucleotides cannot link unless there is some
activation of the
phosphate group, whereas the only effective activating groups for this are "totally implausible in any prebiotic scenario", particularly
adenosine triphosphate.
[57] According to Joyce and Orgel, in case of the phosphate group activation, the basic
polymer product would have
5',5'-pyrophosphate linkages, while the
3',5'-phosphodiester linkages, which are present in all known RNA, would be much less abundant.
[57]
The associated molecules would have been also prone to addition of
incorrect nucleotides or to reactions with numerous other substances
likely to have been present.
[57] The RNA molecules would have been also continuously degraded by such destructive process as spontaneous
hydrolysis, present on the early Earth.
[57] Joyce and Orgel proposed to reject "the myth of a self-replicating RNA molecule that arose
de novo from a soup of random polynucleotides"
[57][citation needed][unreliable source?] and hypothesised a scenario where the prebiotic processes furnish pools of
enantiopure beta-D-ribonucleosides.
[59] In a later paper, Joyce describes "The myth of a small RNA molecule that arises de novo and can replicate
efficiently and with high fidelity under plausible prebiotic conditions" as a
strawman.
[60]
Prebiotic RNA synthesis
Nucleotides
are the fundamental molecules that combine in series to form RNA. They
consist of a nitrogenous base attached to a sugar-phosphate backbone.
RNA is made of long stretches of specific nucleotides arranged so that
their sequence of bases carries information. The RNA world hypothesis
holds that in the
primordial soup (or
sandwich),
there existed free-floating nucleotides. These nucleotides regularly
formed bonds with one another, which often broke because the change in
energy was so low. However, certain sequences of base pairs have
catalytic properties that lower the energy of their chain being created,
enabling them to stay together for longer periods of time. As each
chain grew longer, it attracted more matching nucleotides faster,
causing chains to now form faster than they were breaking down.
These chains have been proposed by some as the first, primitive forms of life.
[61]
In an RNA world, different sets of RNA strands would have had different
replication outputs, which would have increased or decreased their
frequency in the population, i.e.
natural selection.
As the fittest sets of RNA molecules expanded their numbers, novel
catalytic properties added by mutation, which benefitted their
persistence and expansion, could accumulate in the population. Such an
autocatalytic set of ribozymes, capable of self replication in about an hour, has been identified. It was produced by molecular competition (
in vitro evolution) of candidate enzyme mixtures.
[62]
Competition between RNA may have favored the emergence of cooperation
between different RNA chains, opening the way for the formation of the
first
protocell. Eventually, RNA chains developed with catalytic properties that help
amino acids bind together (a process called
peptide-bonding).
These amino acids could then assist with RNA synthesis, giving those
RNA chains that could serve as ribozymes the selective advantage. The
ability to catalyze one step in protein synthesis,
aminoacylation of RNA, has been demonstrated in a short (five-nucleotide) segment of RNA.
[63]
One of the problems with the RNA world hypothesis is to discover the
pathway by which RNA became upgraded to the DNA system. Geoffrey Diemer
and Ken Stedman at Portland State University in Oregon, may have found a
solution. While conducting a survey of viruses in a hot acidic lake in
Lassen Volcanic National Park, California, they uncovered evidence that a
simple DNA virus had acquired a gene from a completely unrelated
RNA-based virus. Virologist Luis Villareal of the University of
California Irvine also suggests that viruses capable of converting an
RNA-based gene into DNA and then incorporating it into a more complex
DNA-based genome might have been common in the Virus world during the
RNA to DNA transition some 4 billion years ago.
[64][65]
This finding bolsters the argument for the transfer of information from
the RNA world to the emerging DNA world before the emergence of the
Last Universal Common Ancestor. From the research, the diversity of this virus world is still with us.
In March 2015, NASA scientists reported that, for the first time, complex DNA and RNA organic compounds of
life, including uracil, cytosine and thymine, have been formed in the laboratory under conditions found only in
outer space, using starting chemicals, like
pyrimidine, found in
meteorites. Pyrimidine, like
polycyclic aromatic hydrocarbons (PAHs), the most carbon-rich chemical found in the
Universe, may have been formed in
giant red stars or in
interstellar dust and gas clouds, according to the scientists.
[66]
Viroids
Additional evidence supporting the concept of an RNA world has resulted from research on
viroids, the first representatives of a novel domain of "subviral pathogens."
[67][68]
Viroids are mostly plant pathogens, which consist of short stretches (a
few hundred nucleobases) of highly complementary, circular,
single-stranded, and non-coding RNA without a protein coat. Compared
with other infectious plant pathogens, viroids are extremely small in
size, ranging from 246 to 467 nucleobases. In comparison, the genome of
the smallest known viruses capable of causing an infection are about
2,000 nucleobases long.
[69]
In 1989, Diener proposed that, based on their characteristic
properties, viroids are more plausible "living relics" of the RNA world
than are
introns or other RNAs then so considered.
[70]
If so, viroids have attained potential significance beyond plant
pathology to evolutionary biology, by representing the most plausible
macromolecules known capable of explaining crucial intermediate steps in
the evolution of life from inanimate matter (see:
abiogenesis).
Apparently, Diener's hypothesis lay dormant until 2014, when Flores
et al. published a review paper, in which Diener's evidence supporting
his hypothesis was summarized.
[71]
In the same year, a New York Times science writer published a
popularized version of Diener's proposal, in which, however, he
mistakenly credited Flores et al. with the hypothesis' original
conception.
[72]
Pertinent viroid properties listed in 1989 are:
- their small size, imposed by error-prone replication;
- their high guanine and cytosine content, which increases stability and replication fidelity;
- their circular structure, which assures complete replication without genomic tags;
- existence of structural periodicity, which permits modular assembly into enlarged genomes;
- their lack of protein-coding ability, consistent with a ribosome-free habitat; and
- replication mediated in some by ribozymes—the fingerprint of the RNA world.[71]
The existence, in extant cells, of RNAs with molecular properties
predicted for RNAs of the RNA World constitutes an additional argument
supporting the RNA World hypothesis.
Origin of sex
Eigen
et al.
[73] and Woese
[74] proposed that the genomes of early
protocells
were composed of single-stranded RNA, and that individual genes
corresponded to separate RNA segments, rather than being linked
end-to-end as in present-day DNA genomes. A protocell that was haploid
(one copy of each RNA gene) would be vulnerable to damage, since a
single lesion in any RNA segment would be potentially lethal to the
protocell (e.g. by blocking replication or inhibiting the function of an
essential gene).
Vulnerability to damage could be reduced by maintaining two or more
copies of each RNA segment in each protocell, i.e. by maintaining
diploidy or polyploidy. Genome redundancy would allow a damaged RNA
segment to be replaced by an additional replication of its homolog.
However, for such a simple organism, the proportion of available
resources tied up in the genetic material would be a large fraction of
the total resource budget. Under limited resource conditions, the
protocell reproductive rate would likely be inversely related to ploidy
number. The protocell's fitness would be reduced by the costs of
redundancy. Consequently, coping with damaged RNA genes while minimizing
the costs of redundancy would likely have been a fundamental problem
for early protocells.
A cost-benefit analysis was carried out in which the costs of
maintaining redundancy were balanced against the costs of genome damage.
[75]
This analysis led to the conclusion that, under a wide range of
circumstances, the selected strategy would be for each protocell to be
haploid, but to periodically fuse with another haploid protocell to form
a transient diploid. The retention of the haploid state maximizes the
growth rate. The periodic fusions permit mutual reactivation of
otherwise lethally damaged protocells. If at least one damage-free copy
of each RNA gene is present in the transient diploid, viable progeny can
be formed. For two, rather than one, viable daughter cells to be
produced would require an extra replication of the intact RNA gene
homologous to any RNA gene that had been damaged prior to the division
of the fused protocell. The cycle of haploid reproduction, with
occasional fusion to a transient diploid state, followed by splitting to
the haploid state, can be considered to be the sexual cycle in its most
primitive form.
[75][76] In the absence of this sexual cycle, haploid protocells with damage in an essential RNA gene would simply die.
This model for the early sexual cycle is hypothetical, but it is very
similar to the known sexual behavior of the segmented RNA viruses,
which are among the simplest organisms known.
Influenza virus, whose genome consists of 8 physically separated single-stranded RNA segments,
[77]
is an example of this type of virus. In segmented RNA viruses, “mating”
can occur when a host cell is infected by at least two virus particles.
If these viruses each contain an RNA segment with a lethal damage,
multiple infection can lead to reactivation providing that at least one
undamaged copy of each virus gene is present in the infected cell. This
phenomenon is known as “multiplicity reactivation”. Multiplicity
reactivation has been reported to occur in influenza virus infections
after induction of RNA damage by
UV-irradiation,
[78] and ionizing radiation.
[79]
Further developments
Patrick Forterre has been working on a novel hypothesis, called "three viruses, three domains":
[80] that viruses were instrumental in the transition from RNA to DNA and the evolution of
Bacteria,
Archaea, and
Eukaryota. He believes the
last common ancestor (specifically, the "last universal cellular ancestor")
[80]
was RNA-based and evolved RNA viruses. Some of the viruses evolved into
DNA viruses to protect their genes from attack. Through the process of
viral infection into hosts the three domains of life evolved.
[80][81] Another interesting proposal is the idea that RNA synthesis might have been driven by temperature gradients, in the process of
thermosynthesis.
[82] Single nucleotides have been shown to catalyze organic reactions.
[83]
Steven Benner has argued that chemical conditions on the planet
Mars, such as the presence of
boron,
molybdenum and
oxygen, may have been better for initially producing RNA molecules than those on
Earth. If so, life-suitable molecules, originating on Mars, may have later migrated to Earth via
panspermia or similar process.
[2][3]
Alternative hypotheses
The
hypothesized existence of an RNA world does not exclude a "Pre-RNA
world", where a metabolic system based on a different nucleic acid is
proposed to pre-date RNA. A candidate nucleic acid is peptide nucleic
acid (
PNA), which uses simple
peptide bonds to link nucleobases.
[84]
PNA is more stable than RNA, but its ability to be generated under
prebiological conditions has yet to be demonstrated experimentally.
Threose nucleic acid (
TNA) has also been proposed as a starting point, as has glycol nucleic acid (
GNA), and like PNA, also lack experimental evidence for their respective abiogenesis.
An alternative — or complementary — theory of RNA origin is proposed in the
PAH world hypothesis, whereby
polycyclic aromatic hydrocarbons (
PAHs) mediate the synthesis of RNA molecules.
[85] PAHs are the most common and abundant of the known polyatomic molecules in the visible
Universe, and are a likely constituent of the
primordial sea.
[86] PAHs, along with
fullerenes (also implicated in the
origin of life),
[87] have been recently detected in
nebulae.
[88]
The
iron-sulfur world theory
proposes that simple metabolic processes developed before genetic
materials did, and these energy-producing cycles catalyzed the
production of genes.
Some of the difficulties of producing the precursors on earth are
bypassed by another alternative or complementary theory for their
origin,
panspermia.
It discusses the possibility that the earliest life on this planet was
carried here from somewhere else in the galaxy, possibly on meteorites
similar to the
Murchison meteorite.
[89]
This does not invalidate the concept of an RNA world, but posits that
this world or its precursors originated not on Earth but rather another,
probably older, planet.
There are hypotheses that are in direct conflict to the RNA world
hypothesis. The relative chemical complexity of the nucleotide and the
unlikelihood of it spontaneously arising, along with the limited number
of combinations possible among four base forms, as well as the need for
RNA polymers of some length before seeing enzymatic activity, have led
some to reject the RNA world hypothesis in favor of a metabolism-first
hypothesis, where the chemistry underlying cellular function arose
first, along with the ability to replicate and facilitate this
metabolism.
RNA-peptide coevolution
Another
proposal is that the dual-molecule system we see today, where a
nucleotide-based molecule is needed to synthesize protein, and a
protein-based molecule is needed to make nucleic acid polymers,
represents the original form of life.
[90] This theory is called RNA-peptide coevolution,
[91]
or the Peptide-RNA world, and offers a possible explanation for the
rapid evolution of high-quality replication in RNA (since proteins are
catalysts), with the disadvantage of having to postulate the formation
of two complex molecules, an enzyme (from peptides) and a RNA (from
nucleotides). In this Peptide-RNA World scenario, RNA would have
contained the instructions for life, while peptides (simple protein
enzymes) would have accelerated key chemical reactions to carry out
those instructions.
[92]
The study leaves open the question of exactly how those primitive
systems managed to replicate themselves — something neither the RNA
World hypothesis nor the Peptide-RNA World theory can yet explain,
unless
polymerases (enzymes that rapidly assemble the RNA molecule) played a role.
[92]
A research project completed in March 2015 by the Sutherland group
found that a network of reactions beginning with hydrogen cyanide and
hydrogen sulfide,
in streams of water irradiated by UV light, could produce the chemical
components of proteins and lipids, alongside those of RNA.
[93][94] The researchers used the term "cyanosulfidic" to describe this network of reactions.
[93]
Implications of the RNA world
The RNA world hypothesis, if true, has important implications for the
definition of life. For most of the time that followed
Watson and
Crick's
elucidation of DNA structure in 1953, life was largely defined in terms
of DNA and proteins: DNA and proteins seemed the dominant
macromolecules in the living cell, with RNA only aiding in creating
proteins from the DNA blueprint.
The RNA world hypothesis places RNA at center-stage when life originated. This has been accompanied by many studies
[citation needed]
in the last ten years that demonstrate important aspects of RNA
function not previously known—and supports the idea of a critical role
for RNA in the mechanisms of life. The RNA world hypothesis is supported
by the observations that ribosomes are ribozymes: the catalytic site is
composed of RNA, and proteins hold no major structural role and are of
peripheral functional importance. This was confirmed with the
deciphering of the 3-dimensional structure of the ribosome in 2001.
Specifically, peptide bond formation, the reaction that binds
amino acids together into
proteins, is now known to be catalyzed by an adenine residue in the
rRNA.
Other interesting discoveries demonstrate a role for RNA beyond a simple message or transfer molecule.
[95] These include the importance of
small nuclear ribonucleoproteins (snRNPs) in the processing of
pre-mRNA and
RNA editing,
RNA interference (RNAi), and
reverse transcription from RNA in eukaryotes in the maintenance of
telomeres in the
telomerase reaction.
[96]