 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sativex (with THC), Epidiolex |
| Synonyms | CBD, cannabidiolum, (−)-cannabidiol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Inhalation (smoking, vaping), buccal (aerosol spray), oral (solution) |
| Drug class | Cannabinoid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | • Oral: 13–19% • Inhaled: 31% (11–45%) |
| Elimination half-life | 18–32 hours |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ECHA InfoCard | 100.215.986 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.464 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 66 °C (151 °F) |
Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of some 113 identified cannabinoids in cannabis plants, accounting for up to 40% of the plant's extract. As of 2018, preliminary clinical research on cannabidiol included studies of anxiety, cognition, movement disorders, and pain.
Cannabidiol can be taken into the body in multiple ways, including by inhalation of cannabis smoke or vapor, as an aerosol spray into the cheek, and by mouth. It may be supplied as CBD oil containing only CBD as the active ingredient (no added tetrahydrocannabinol [THC] or terpenes), a full-plant CBD-dominant hemp extract oil, capsules, dried cannabis, or as a prescription liquid solution. CBD does not have the same psychoactivity as THC, and may affect the actions of THC. Although in vitro studies indicate CBD may interact with different biological targets, including cannabinoid receptors and other neurotransmitter receptors, as of 2018 the mechanism of action for its biological effects has not been determined.
In the United States, the cannabidiol drug Epidiolex has been approved by the Food and Drug Administration for treatment of two epilepsy disorders. The side effects of long-term use of the drug include somnolence, decreased appetite, diarrhea, fatigue, malaise, weakness, sleeping problems, and others.
The U.S. Drug Enforcement Administration has assigned Epidiolex a Schedule V classification, while non-Epidiolex CBD remains a Schedule I drug prohibited for any use. Cannabidiol is not scheduled under any United Nations drug control treaties, and in 2018 the World Health Organization recommended that it remain unscheduled.
Medical uses
Epilepsy
There has been little high-quality research into the use of cannabidiol for epilepsy, and what there is is limited to refractory epilepsy in children.
While the results of using medical-grade cannabidiol in combination
with conventional medication shows some promise, they did not lead to
seizures being eliminated, and were associated with some minor adverse effects.
An orally administered cannabidiol solution (brand name
Epidiolex) was approved by the US Food and Drug Administration in June
2018 as a treatment for two rare forms of childhood epilepsy, Lennox-Gastaut syndrome and Dravet syndrome.
Other uses
Preliminary research on other possible therapeutic uses for cannabidiol include several neurological disorders, but the findings have not been confirmed by sufficient high-quality clinical research to establish such uses in clinical practice.
Side effects
Preliminary research indicates that cannabidiol may reduce adverse effects of THC, particularly those causing intoxication and sedation, but only at high doses. Safety studies of cannabidiol showed it is well-tolerated, but may cause tiredness, diarrhea, or changes in appetite as common adverse effects. Epidiolex documentation lists sleepiness, insomnia and poor quality sleep, decreased appetite, diarrhea, and fatigue.
Potential interactions
Laboratory evidence indicated that cannabidiol may reduce THC clearance, increasing plasma concentrations which may raise THC availability to receptors and enhance its effect in a dose-dependent manner. In vitro, cannabidiol inhibited receptors affecting the activity of voltage-dependent sodium and potassium channels, which may affect neural activity. A small clinical trial reported that CBD partially inhibited the CYP2C-catalyzed hydroxylation of THC to 11-OH-THC.
Pharmacology
Pharmacodynamics
Cannabidiol has very low affinity for the cannabinoid CB1 and CB2 receptors but is said to act as an indirect antagonist of these receptors. At the same time, it may potentiate the effects of THC by increasing CB1 receptor density or through another CB1 receptor-related mechanism.
Cannabidiol has been found to act as an antagonist of GPR55, a G protein-coupled receptor and putative cannabinoid receptor that is expressed in the caudate nucleus and putamen in the brain. It has also been found to act as an inverse agonist of GPR3, GPR6, and GPR12. Although currently classified as orphan receptors, these receptors are most closely related phylogenetically to the cannabinoid receptors. In addition to orphan receptors, CBD has been shown to act as a serotonin 5-HT1A receptor partial agonist, and this action may be involved in its antidepressant, anxiolytic, and neuroprotective effects. It is an allosteric modulator of the μ- and δ-opioid receptors as well. The pharmacological effects of CBD have additionally been attributed to PPARγ agonism and intracellular calcium release.
Research suggests that CBD may exert some of its pharmacological action through its inhibition of fatty acid amide hydrolase (FAAH), which may in turn increase the levels of endocannabinoids, such as anandamide, produced by the body.
It has also been speculated that some of the metabolites of CBD have
pharmacological effects that contribute to the biological activity of
CBD.
Pharmacokinetics
The oral bioavailability of CBD is 13 to 19%, while its bioavailability via inhalation is 11 to 45% (mean 31%). The elimination half-life of CBD is 18–32 hours.
Cannabidiol is metabolized in the liver as well as in the intestines by CYP2C19 and CYP3A4 enzymes, and UGT1A7, UGT1A9, and UGT2B7 isoforms.
Pharmaceutical preparations
Nabiximols (brand name Sativex) is a patented medicine containing CBD and THC in equal proportions.
The drug was approved by Health Canada in 2005 for prescription to treat central neuropathic pain in multiple sclerosis, and in 2007 for cancer related pain.
In New Zealand Sativex® is approved for use as an add-on treatment for
symptom improvement in patients with moderate to severe spasticity due
to Multiple Sclerosis who have not responded adequately to other
anti-spasticity medication and who demonstrate clinically significant
improvement in spasticity related symptoms during an initial trial of
therapy.
Chemistry
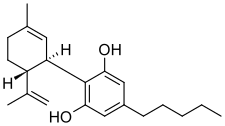
Cannabidiol is insoluble in water but soluble in organic solvents such as pentane. At room temperature, it is a colorless crystalline solid. In strongly basic media and the presence of air, it is oxidized to a quinone. Under acidic conditions it cyclizes to THC, which also occurs during pyrolysis (smoking). The synthesis of cannabidiol has been accomplished by several research groups.
Biosynthesis
Cannabidiol and THC biosynthesis
Cannabis produces CBD-carboxylic acid through the same metabolic pathway as THC, until the next to last step, where CBDA synthase performs catalysis instead of THCA synthase.
Isomerism
History
CBD was isolated from the cannabis plant in 1940, and its chemical structure was established in 1963.
Society and culture
Names
Food and beverage
An example of CBD-infused cold brewed coffee; tea on a grocery store shelf.
Food and beverage products containing CBD were introduced in the United States in 2017.
Similar to energy drinks and protein bars
which may contain vitamin or herbal additives, food and beverage items
can be infused with CBD as an alternative means of ingesting the
substance. In the United States, numerous products are marketed as containing CBD, but in reality contain little or none. Some companies marketing CBD-infused food products with claims that are similar to the effects of prescription drugs have received warning letters from the Food and Drug Administration for making unsubstantiated health claims. In February 2019, the New York City Department of Health announced plans to fine restaurants that sell food or drinks containing CBD, beginning in October 2019.
Plant sources
Selective
breeding of cannabis plants has expanded and diversified as commercial
and therapeutic markets develop. Some growers in the U.S. succeeded in
lowering the proportion of CBD-to-THC to accommodate customers who
preferred varietals that were more mind-altering due to the higher THC
and lower CBD content.
In the USA, hemp is classified by the federal government as cannabis
containing no more than 0.3% THC by dry weight. This classification was
established in the 2018 Farm Bill and was refined to include
hemp-sourced extracts, cannabinoids, and derivatives in the definition
of hemp.
Legal status
Non-psychoactivity
CBD
does not appear to have any psychotropic ("high") effects such as those
caused by ∆9-THC in marijuana, but may have anti-anxiety and
anti-psychotic effects.
As the legal landscape and understanding about the differences in
medical cannabinoids unfolds, experts are working to distinguish
"medical marijuana" (with varying degrees of psychotropic effects and
deficits in executive function) – from "medical CBD therapies” which
would commonly present as having a reduced or non-psychoactive
side-effect profile.
Various strains of "medical marijuana" are found to have a
significant variation in the ratios of CBD-to-THC, and are known to
contain other non-psychotropic cannabinoids. Any psychoactive marijuana, regardless of its CBD content, is derived from the flower (or bud) of the genus Cannabis. Non-psychoactive hemp (also commonly-termed industrial hemp),
regardless of its CBD content, is any part of the cannabis plant,
whether growing or not, containing a ∆-9 tetrahydrocannabinol
concentration of no more than 0.3% on a dry-weight basis.
Certain standards are required for legal growing, cultivating, and
producing the hemp plant. The Colorado Industrial Hemp Program registers
growers of industrial hemp and samples crops to verify that the
dry-weight THC concentration does not exceed 0.3%.
United Nations
Cannabidiol is not scheduled under the Convention on Psychotropic Substances or any other UN drug treaty. In 2018, the World Health Organization recommended that CBD remain unscheduled.
United States
In
the United States, CBD's legal status depends on the source from which
it is derived. When derived from marijuana it is a schedule 1 controlled
substance under the federal Controlled Substances Act
(CSA). This is because the CSA's definition of marijuana (spelled
"marihuana") includes "all parts" of the cannabis plant. When it is the
investigative new drug Epidiolex it is schedule 5 (see below). However,
when CBD is derived from hemp or some other lawful source it is not a
controlled substance. Section 10113 of the Agricultural Improvement Act of 2018 defines "hemp"
as "the plant Cannabis sativa L. and any part of that plant, including
the seeds thereof and all derivatives, extracts, cannabinoids, isomers,
acids, salts, and salts of isomers, whether growing or not, with a
delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent
on a dry weight basis." Hemp is excluded from the definition of
marijuana under the Controlled Substances Act
(CSA). CBD is not specifically scheduled in the CSA. It is therefore
lawful when derived from hemp, which is not a controlled substance and
the definition of which includes "cannabinoids". CBD is a cannabinoid.
In September 2018, following its approval by the FDA for rare types of childhood epilepsy, Epidiolex was rescheduled (by the Drug Enforcement Administration) as a Schedule V drug to allow for its prescription use. This change applies only to FDA-approved products containing no more than 0.1 percent THC. This allows GW Pharmaceuticals to sell Epidiolex, but it does not apply broadly and all other CBD-containing products remain Schedule I drugs. Epidiolex still requires rescheduling in some states before it can be prescribed in those states.
A CNN program that featured Charlotte's Web cannabis in 2013 brought increased attention to the use of CBD in the treatment of seizure disorders.
Since then, 16 states have passed laws to allow the use of CBD
products with a doctor's recommendation (instead of a prescription) for
treatment of certain medical conditions. This is in addition to the 30 states
that have passed comprehensive medical cannabis laws, which allow for
the use of cannabis products with no restrictions on THC content.
Of these 30 states, eight have legalized the use and sale of cannabis
products without requirement for a doctor's recommendation.
Some manufacturers ship CBD products nationally, an illegal
action which the FDA has not enforced in 2018, with CBD remaining the
subject of an FDA investigational new drug evaluation, and is not considered legal as a dietary supplement or food ingredient as of December 2018. Federal illegality has made it difficult historically to conduct research on CBD. CBD is openly sold in head shops and health food stores in some states where such sales have not been explicitly legalized.
The 2014 Farm Bill legalized the sale of "non-viable hemp material" grown within states participating in the Hemp Pilot Program. This legislation defined hemp as cannabis containing less than 0.3% of THC delta-9, grown within the regulatory framework of the Hemp Pilot Program. The 2018 Farm Bill allowed for interstate commerce of hemp derived products, though these products still fall under the purview of the FDA.
Australia
Prescription
medicine (Schedule 4) for therapeutic use containing 2 per cent (2.0%)
or less of other cannabinoids commonly found in cannabis (such as
∆9-THC). A schedule 4 drug under the SUSMP
is Prescription Only Medicine, or Prescription Animal Remedy –
Substances, the use or supply of which should be by or on the order of
persons permitted by State or Territory legislation to prescribe and
should be available from a pharmacist on prescription.
Following a change in legislation in 2017, CBD was changed from a
schedule 9 drug to a schedule 4 drug, meaning that it is legally
available in Australia.
New Zealand
The
passing of the Misuse of Drugs (Medicinal Cannabis) Amendment Act in
December 2018 means some products containing cannabidiol (CBD) are now
prescription medicines only.
Cannabidiol is no longer a controlled drug in New Zealand under
the Misuse of Drugs Act. It is a prescription medicine under the
Medicines Act provided the product contains no more than two percent THC
of total CBD.
In 2017 under the previous government, Associate Health Minister Peter Dunne
had made changes to the regulations so that restrictions would be
removed, which meant a doctor was able to prescribe cannabidiol to
patients.
Canada
On October 17, 2018, cannabidiol became legal for recreational and medical use.
European Union
In 2019, the European Commission announced that CBD and other cannabinoids would be classified as "novel foods", meaning that CBD products would require authorization under the EU Novel Food Regulation
stating: because "this product was not used as a food or food
ingredient before 15 May 1997, before it may be placed on the market in
the EU as a food or food ingredient, a safety assessment under the Novel
Food Regulation is required."
The recommendation – applying to CBD extracts, synthesized CBD, and all
CBD products, including CBD oil – was scheduled for a final ruling by
the European Commission in March 2019.
If approved, manufacturers of CBD products would be required to conduct
safety tests and prove safe consumption, indicating that CBD products
would not be eligible for legal commerce until at least 2021.
Cannabidiol is listed in the EU Cosmetics Ingredient Database (CosIng). However, the listing of an ingredient, assigned with an INCI name, in CosIng does not mean it is to be used in cosmetic products or is approved for such use.
Several industrial hemp varieties can be legally cultivated in Western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1%, with THC less than 0.1%.
Sweden
CBD is classified as a medical product in Sweden.
United Kingdom
Cannabidiol,
in an oral-mucosal spray formulation combined with
delta-9-tetrahydrocannabinol, is a product available (by prescription
only until 2017) for relief of severe spasticity due to multiple
sclerosis (where other anti-spasmodics have not been effective).
Until 2017, products containing cannabidiol marketed for medical purposes were classed as medicines by the UK regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA) and could not be marketed without regulatory approval for the medical claims. As of 2018,
cannabis oil is legal to possess, buy, and sell in the UK, providing
the product does not contain more than 0.2% THC and is not advertised as
providing a medicinal benefit.
In January 2019, the UK Food Standards Agency indicated it would regard CBD products, including CBD oil, as a novel food
in the UK, having no history of use before May 1997, and indicating
such products must have authorization and proven safety before being
marketed.
Switzerland
While
THC remains illegal, CBD is not subject to the Swiss Narcotic Acts
because this substance does not produce a comparable psychoactive
effect. Cannabis products containing less than 1% THC can be sold and purchased legally.