|
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(2R,3R,4S,5R)-5-(hydroxymethyl)oxolane-2,3,4-triol
| |||
| Other names
d-Ribose
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEMBL | |||
| ChemSpider |
| ||
| DrugBank | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| Properties | |||
| C5H10O5 | |||
| Molar mass | 150.13 | ||
| Appearance | White solid | ||
| Melting point | 95 °C (203 °F; 368 K) | ||
| 100 g/L (25 °C, 77 °F) | |||
Chiral rotation ([α]D)
|
−21.5° (H2O) | ||
| Related compounds | |||
Related aldopentoses
|
Arabinose Xylose Lyxose | ||
Related compounds
|
Deoxyribose | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
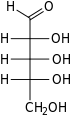
L-Ribose Fischer Projection
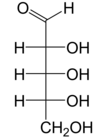
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, d-ribose, is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation and expression of genes. It has a structural analog, deoxyribose, which is a similarly essential component of DNA. l-Ribose is an unnatural sugar that was first prepared by Emil Fischer and Oscar Piloty in 1891. It was not until 1909 that Phoebus Levene and Walter Jacobs recognised that d-ribose was a natural product, the enantiomer of Fischer and Piloty's product, and an essential component of nucleic acids. Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer at the 2' carbon; both names also relate to gum arabic, from which arabinose was first isolated and from which they prepared l-ribose.
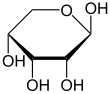
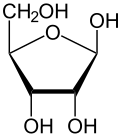
Top: Haworth projections of one of each of the furanose and pyranose forms of d-ribose
Bottom: Fischer projection of the open chain forms of d- and l-ribose
Bottom: Fischer projection of the open chain forms of d- and l-ribose
Like most sugars, ribose exists as a mixture of cyclic forms in equilibrium with its linear form, and these readily interconvert especially in aqueous solution.
The name "ribose" is used in biochemistry and biology to refer to all
of these forms, though more specific names for each are used when
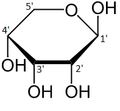
required. In its linear form, ribose can be recognised as the pentose sugar with all of its hydroxyl functional groups on the same side in its Fischer projection. d-Ribose has these hydroxyl groups on the right hand side and is associated with the systematic name (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal, whilst l-ribose has its hydroxyl groups appear on the left hand side in a Fischer projection. Cyclisation of ribose occurs via hemiacetal formation due to attack on the aldehyde by the C4' hydroxyl group to produce a furanose form or by the C5' hydroxyl group to produce a pyranose form. In each case, there are two possible geometric outcomes, named as α- and β- and known as anomers, depending on the stereochemistry at the hemiacetal carbon atom (the "anomeric carbon"). At room temperature, about 76% of d-ribose is present in pyranose forms (α:β = 1:2)[10] and 24% in the furanose forms (α:β = 1:3), with only about 0.1% of the linear form present.
The ribonucleosides adenosine, cytidine, guanosine, and uridine are all derivatives of β-d-ribofuranose. Metabolically-important species that include phosphorylated ribose include ADP, ATP, coenzyme A, and NADH. cAMP and cGMP serve as secondary messengers in some signaling pathways and are also ribose derivatives. The ribose moiety appears in some pharmaceutical agents, including the antibiotics neomycin and paromomycin.
Sources
In November 2019, scientists reported detecting, for the first time, sugar molecules, including ribose, in meteorites, suggesting that chemical processes on asteroids can produce some fundamentally essential bio-ingredients important to life, and supporting the notion of an RNA world prior to a DNA-based origin of life on Earth, and possibly, as well, the notion of panspermia.
Structure
Ribose is an aldopentose (a monosaccharide containing five carbon atoms) that, in its open chain form, has an aldehyde functional group
at one end. In the conventional numbering scheme for monosaccharides,
the carbon atoms are numbered from C1' (in the aldehyde group) to C5'.
The deoxyribose derivative found in DNA differs from ribose by having a hydrogen atom in place of the hydroxyl group at C2'. This hydroxyl group performs a function in RNA splicing.
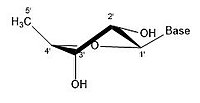
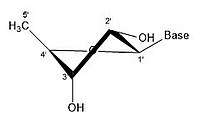
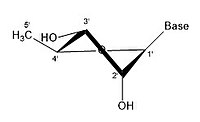
Like many monosaccharides, ribose exists in an equilibrium among 5 forms—the linear form H−(C=O)−(CHOH)4–H and either of the two ring forms: α- or β-ribofuranose ("C3'-endo"), with a five-membered tetrahydrofuran ring, and α- or β-ribopyranose ("C2'-endo"), with a six-membered tetrahydropyran ring.
The “d-” in the name d-ribose refers to the stereochemistry of the chiral carbon atom farthest away from the aldehyde group (C4'). In d-ribose, as in all d-sugars, this carbon atom has the same configuration as in d-glyceraldehyde.
Relative abundance of different forms of ribose in solution: β-d-ribopyranose (59%), α-d-ribopyranose (20%), β-d-ribofuranose (13%), α-d-ribofuranose (7%) and open chain (0.1%).
When ribose sugars are in nucleosides and nucleotide,
the torsion angles for the rotation encompassing the bonds influence
the configuration of the respective nucleoside and nucleotide. The secondary structure of a nucleic acid is determined by the rotation of its 7 torsion angles.
Having a large amount of torsion angles allows for greater flexibility
of a molecule. However, because these sugars have a higher degree of
flexibility, it leads to many issues when simulating conditions for RNA
molecules because there are so many different conformations of ribose.
In closed ring riboses, the observed flexibility mentioned above is not
observed because the ring cycle imposes a limit on the number of torsion
angles possible in the structure. There are different conformations of closed form riboses. These differ in regards to how the lone oxygen in the molecule is positioned respective to the nitrogenous base (also known as a nucleobase
or just a base) attached to the ribose. If a carbon is facing towards
the base, then the ribose is labeled as endo. If a carbon is facing away
from the base, then the ribose is labeled as exo. If there is an oxygen
molecule attached to the 2' carbon of a closed cycle ribose, then the
exo confirmation is more stable because it decreases the interactions of
the oxygen with the base.
The difference itself is quite small, but when looking at an entire
chain of RNA the slight difference amounts to a sizable impact.
- Different pucker configurations of Ribose
A ribose molecule is typically represented as a planar molecule
on paper. Despite this, it is typically non-planar in nature. Even
between hydrogen atoms, the many constituents on a ribose molecule cause
steric hindrance and strain between them. To relieve this crowding and ring strain, the ring puckers, i.e. becomes non-planar. This puckering is achieved by displacing an atom from the plane, relieving the strain and yielding a more stable configuration.
Puckering, otherwise known as the sugar ring conformation (specifically
ribose sugar), can be described by the amplitude of pucker as well as
the pseudorotation
angle. The pseudo-rotation angle can be described as either "north (N)”
or "south (S)” range. While both ranges are found in double helices,
the north range is commonly associated with RNA and the A form of DNA. In contrast, the south range is associated with B form DNA. Z-DNA contains sugars in both the north and south ranges.
When only a single atom is displaced, it is referred to as an
"envelope" pucker. When two atoms are displaced, it is referred to as a
"twist" pucker, in reference to the zigzag orientation.
In an "endo" pucker, the major displacement of atoms is on the β-face,
the same side as the C4'-C5' bond and the base. In an "exo" pucker, the
major displacement of atoms is on the α-face, on the opposite side of
the ring. The major forms of ribose are the 3'-endo pucker (commonly
adopted by RNA and A-form DNA) and 2'-endo pucker (commonly adopted by
B-form DNA).
These ring puckers are developed from changes in ring torsion angles;
there are infinite combinations of angles so therefore, there is an
infinite number of transposable pucker conformations, each separated by
different activation energies.
Functions in Biochemistry
Ribose
plays many important roles in metabolism, which means that it is
involved in a lot of biochemistry. Ribose is used as a building block
for a lot of the signals and products throughout the metabolic pathway.
One of the most important products of the metabolic pathway is adenosine triphosphate (ATP), which provides energy that drives processes in cells. ATP is derived from ribose; it contains one ribose, three phosphate groups, and an adenine base. ATP is created during cellular respiration from adenosine diphosphate (ATP with one less phosphate group).
Signaling pathways
Ribose also plays a major role in signaling pathways because it is a building block in secondary signaling molecules such as cyclic adenosine monophosphate (cAMP) which is derived from ATP. One specific case in which cAMP is used is in cAMP-dependent signaling pathways. In cAMP signaling pathways, either a stimulative or inhibitory hormone receptor is activated by a signal molecule. These receptors are linked to a stimulative or inhibitory regulative G-protein. When a stimulative G-protein is activated, adenylyl cyclase catalyzes ATP into cAMP by using Mg2+ or Mn2+. cAMP, a secondary messenger, then goes on to activate protein kinase A, which is an enzyme that regulates cell metabolism. Protein kinase A regulates metabolic enzymes by phosphorylation
which causes a change in the cell depending on the original signal
molecule. The opposite occurs when an inhibitory G-protein is activated;
the G-protein inhibits adenylyl cyclase and ATP is not converted to
cAMP.
The difference between ribose and deoxyribose is the presence of a 2'OH
DNA vs RNA
DNA
contains a deoxygenated form of ribose called deoxyribose. The
difference between ribose and deoxyribose is the presence of an OH group
on the 2' carbon of the ring in ribose, making ribose less stable than
deoxyribose and more susceptible to hydrolysis.
RNA
hydrolysis mechanism: The 2' OH in RNA can be hydrolyzed causing RNA to
be less stable than DNA. Hydrolysis of RNA leads to a break in the
backbone of RNA.
Metabolism
Ribose is referred to as the "molecular currency" because of its involvement in intracellular energy transfers. For example, nicotinamide adenine dinucleotide (NAD), flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide phosphate (NADP) all contain the d-ribofuranose moiety. They can each be derived from d-ribose after it is converted to d-ribose 5-phosphate by the enzyme ribokinase. NAD, FAD, and NADP act as electron acceptors in biochemical redox reactions in major metabolic pathways including glycolysis, the citric acid cycle, fermentation, and the electron transport chain.
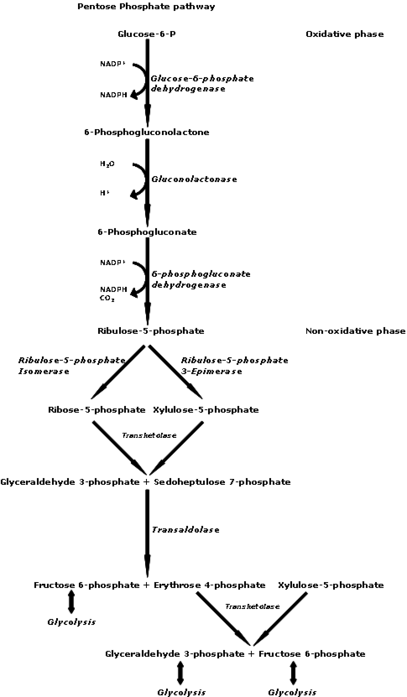
Pentose Phosphate Pathway: begins with d-glucose and includes d-ribose 5-phosphate as an intermediate
Pentose phosphate pathway
d-Ribose 5-phosphate is also produced from d-glucose via the pentose phosphate pathway and is then used to make nucleotides for RNA and DNA.
Nucleotides are synthesized through salvage or de novo synthesis. Nucleotide salvage
uses pieces of previously made nucleotides and re-synthesizes them for
future use. In de novo, amino acids, carbon dioxide, folate derivatives,
and phosphoribosyl pyrophosphate (PRPP) are used to synthesize nucleotides. Both de novo and salvage require PRPP which is synthesized from ATP and ribose 5-phosphate by an enzyme called PRPP synthetase.
Modifications
Modifications in nature
In biology, d-ribose must be phosphorylated by the cell before it can be used. Ribokinase catalyzes this reaction by converting d-ribose to d-ribose 5-phosphate. Once converted, d-ribose-5-phosphate is available for the manufacturing of the amino acids tryptophan and histidine, or for use in the pentose phosphate pathway. The absorption of d-ribose is 88–100% in the small intestines (up to 200 mg/kg·h).
In addition to phosphorylation, there are other modifications.
One important modification occurs at the C2' position of the ribose
molecule. By adding an O-alkyl
group, the nuclear resistance of the RNA is increased because of
additional stabilizing forces. These forces are stabilizing because of
the increase of intramolecular hydrogen bonding and an increase in the glycosidic bond stability. The resulting increase of resistance leads to increases in the half-life of siRNA and the potential therapeutic potential in cells and animals. The methylation of ribose at particular sites is correlated with a decrease in immune stimulation.
Synthetic modifications
Along with phosphorylation, ribofuranose molecules can exchange their oxygen with selenium and sulfur
to produce similar sugars that only vary at the 4' position. These
substitutions are very notable, because the molecules produced are more lipophilic
than the original molecule. The increase in lipophilicity makes the new
molecules more suitable subjects for use in techniques such as PCR, RNA aptamer post-modifcation, antisense technology, and for phasing X-ray crystallographic data.
Similar to the 2' modifications in nature, a synthetic modification of ribose includes the addition of fluorine at the 2' position. This fluorinated
ribose acts similar to the methylated ribose because it is capable of
suppressing immune stimulation depending on the location of the ribose
in the DNA strand.
The big difference between methylation and fluorination, is the latter
only occurs through synthetic modifications. The addition of Fluorine
leads to an increase in the stabilization of the glycosidic bond and an
increase of intramolecular hydrogen bonds.
Medical uses
d-ribose has been suggested for use in management of congestive heart failure (as well as other forms of heart disease) and for chronic fatigue syndrome
(CFS), also called myalgic encephalomyelitis (ME) in an open-label
non-blinded, non-randomized, and non-crossover subjective study.
Supplemental d-ribose can bypass part of the pentose phosphate pathway, an energy-producing pathway, to produce d-ribose-5-phosphate. The enzyme glucose-6-phosphate-dehydrogenase (G-6-PDH) is often in short supply in cells, but more so in diseased tissue, such as in myocardial cells in patients with cardiac disease. The supply of d-ribose in the mitochondria is directly correlated with ATP production; decreased d-ribose supply reduces the amount of ATP being produced. Studies suggest that supplementing d-ribose
following tissue ischemia (e.g. myocardial ischemia) increases
myocardial ATP production, and therefore mitochondrial function.
Essentially, administering supplemental d-ribose bypasses an enzymatic step in the pentose phosphate pathway by providing an alternate source of 5-phospho-d-ribose 1-pyrophosphate for ATP production. Supplemental d-ribose
enhances recovery of ATP levels while also reducing cellular injury in
humans and other animals. One study suggested that the use of
supplemental d-ribose reduces the instance of angina in men with diagnosed coronary artery disease. d-Ribose has been used to treat many pathological conditions, such as chronic fatigue syndrome, fibromyalgia,
and myocardial dysfunction. It is also used to reduce symptoms of
cramping, pain, stiffness, etc after exercise and to improve athletic
performance.
Risks
Oral D-Ribose intake is linked to memory loss, anxiety, & Aβ-like deposits associated with Alzheimer’s in mice.