Recent human evolution refers to evolutionary adaptation, sexual and natural selection, and genetic drift within Homo sapiens populations, since their separation and dispersal in the Middle Paleolithic about 50,000 years ago. Contrary to popular belief, not only are humans still evolving, their evolution since the dawn of agriculture is faster than ever before. It is possible that human culture—itself a selective force—has accelerated human evolution. With a sufficiently large data set and modern research methods, scientists can study the changes in the frequency of an allele occurring in a tiny subset of the population over a single lifetime, the shortest meaningful time scale in evolution. Comparing a given gene with that of other species enables geneticists to determine whether it is rapidly evolving in humans alone. For example, while human DNA is on average 98% identical to chimp DNA, the so-called Human Accelerated Region 1 (HAR1), involved in the development of the brain, is only 85% similar.
Following the peopling of Africa some 130,000 years ago, and the recent Out-of-Africa expansion some 70,000 to 50,000 years ago, some sub-populations of Homo sapiens have been geographically isolated for tens of thousands of years prior to the early modern Age of Discovery. Combined with archaic admixture, this has resulted in significant genetic variation, which in some instances has been shown to be the result of directional selection taking place over the past 15,000 years, which is significantly later than possible archaic admixture events. That the human populations living on different parts of the globe have been evolving on divergent trajectories reflects the different conditions of their habitats. Selection pressures were especially severe for populations affected by the Last Glacial Maximum (LGM) in Eurasia, and for sedentary farming populations since the Neolithic, or New Stone Age.
Single nucleotide polymorphisms (SNP, pronounced 'snip'), or mutations of a single genetic code "letter" in an allele that spread across a population, in functional parts of the genome can potentially modify virtually any conceivable trait, from height and eye color to susceptibility to diabetes and schizophrenia. Approximately 2% of the human genome codes for proteins and a slightly larger fraction is involved in gene regulation. But most of the rest of the genome has no known function. If the environment remains stable, the beneficial mutations will spread throughout the local population over many generations until it becomes a dominant trait. An extremely beneficial allele could become ubiquitous in a population in as little as a few centuries whereas those that are less advantageous typically take millennia.
Human traits that emerged recently include the ability to free-dive for long periods of time, adaptations for living in high altitudes where oxygen concentrations are low, resistance to contagious diseases (such as malaria), fair skin, blue eyes, lactase persistence (or the ability to digest milk after weaning), lower blood pressure and cholesterol levels, thick hair shaft, dry ear wax, lower chances of drunkenness, higher body-mass index, reduced prevalence of Alzheimer's disease, lower susceptibility to diabetes, genetic longevity, shrinking brain sizes, and changes in the timing of menarche and menopause.
Archaic admixture
Genetic evidence suggests that a species dubbed Homo heidelbergensis is the last common ancestor of Neanderthals, Denisovans, and Homo sapiens.
This common ancestor lived between 600,000 and 750,000 years ago,
likely in either Europe or Africa. Members of this species migrated
throughout Europe, the Middle East, and Africa and became the
Neanderthals in Western Asia and Europe while another group moved
further east and evolved into the Denisovans, named after the Denisovan
Cave in Russia where the first known fossils of them were discovered. In
Africa, members this group eventually became anatomically modern
humans. Migrations and geographical isolation notwithstanding, the three
descendant groups of Homo heidelbergensis later met and interbred.
DNA analysis reveals that modern-day Tibetans, Melanesians, and Australian Aboriginals carry about 3%-5% of Denisovan DNA. In addition, DNA analysis of Indonesians and Papua New Guineans indicates that Homo sapiens and Denisovans interbred as recently as between 15,000 and 30,000 years ago.
Archaeological research suggests that as prehistoric humans swept across Europe 45,000 years ago, Neanderthals went extinct. Even so, there is evidence of interbreeding between the two groups as humans expanded their presence in the continent. While prehistoric humans carried 3%-6% Neanderthal DNA, modern humans have only about 2%. This seems to suggest selection against Neanderthal-derived traits. For example, the neighborhood of the gene FOXP2, affecting speech and language, shows no signs of Neanderthal inheritance whatsoever.
Introgression of genetic variants acquired by Neanderthal admixture has different distributions in Europeans and East Asians, pointing to differences in selective pressures. Though East Asians inherit more Neanderthal DNA than Europeans, East Asians, South Asians, and Europeans all share Neanderthal DNA, so hybridization likely occurred between Neanderthals and their common ancestors coming out of Africa. Their differences also suggest separate hybridization events for the ancestors of East Asians and other Eurasians.
Following the genome sequencing of three Vindija Neanderthals, a draft sequence of the Neanderthal genome was published and revealed that Neanderthals shared more alleles with Eurasian populations—such as French, Han Chinese, and Papua New Guinean—than with sub-Saharan African populations, such as Yoruba and San. According to the authors of the study, the observed excess of genetic similarity is best explained by recent gene flow from Neanderthals to modern humans after the migration out of Africa. But gene flow did not go one way. The fact that some of the ancestors of modern humans in Europe migrated back into Africa means that modern Africans also carry some genetic materials from Neanderthals. In particular, Africans share 7.2% Neanderthal DNA with Europeans but only 2% with East Asians.
Some climatic adaptations, such as high-altitude adaptation in humans, are thought to have been acquired by archaic admixture. An ethnic group known as the Sherpas from Nepal is believed to have inherited an allele called EPAS1, which allows them to breathe easily at high altitudes, from the Denisovans. A 2014 study reported that Neanderthal-derived variants found in East Asian populations showed clustering in functional groups related to immune and haematopoietic pathways, while European populations showed clustering in functional groups related to the lipid catabolic process. A 2017 study found correlation of Neanderthal admixture in modern European populations with traits such as skin tone, hair color, height, sleeping patterns, mood and smoking addiction. A 2020 study of Africans unveiled Neanderthal haplotypes, or alleles that tend to be inherited together, linked to immunity and ultraviolet sensitivity. The promotion of beneficial traits acquired from admixture is known as adaptive introgression.
Upper Paleolithic, or the Late Stone Age (50,000 to 12,000 years ago)
DNA analyses conducted since 2007 revealed the acceleration of evolution with regards to defenses against disease, skin color, nose shapes, hair color and type, and body shape since about 40,000 years ago, continuing a trend of active selection since humans emigrated from Africa 100,000 years ago. Humans living in colder climates tend to be more heavily built compared to those in warmer climates because having a smaller surface area compared to volume makes it easier to retain heat. People from warmer climates tend to have thicker lips, which have large surface areas, enabling them to keep cool. With regards to nose shapes, humans residing in hot and dry places tend to have narrow and protruding noses in order to reduce loss of moisture. Humans living in hot and humid places tend to have flat and broad noses that moisturizes inhaled hair and retains moisture from exhaled air. Humans dwelling in cold and dry places tend to have small, narrow, and long noses in order to warm and moisturize inhaled air. As for hair types, humans from regions with colder climates tend to have straight hair so that the head and neck are kept warm. Straight hair also allows cool moisture to quickly fall off the head. On the other hand, tight and curly hair increases the exposed areas of the scalp, easing the evaporation of sweat and allowing heat to be radiated away while keeping itself off the neck and shoulders. Epicanthic eye folds are believed to be an adaptation protecting the eye from the snow and reducing snow glare.
Physiological or phenotypical changes have been traced to Upper Paleolithic mutations, such as the East Asian variant of the EDAR gene, dated to about 35,000 years ago. Traits affected by the mutation are sweat glands, teeth, hair thickness and breast tissue. While Africans and Europeans carry the ancestral version of the gene, most East Asians have the mutated version. By testing the gene on mice, Yana G. Kamberov and Pardis C. Sabeti and their colleagues at the Broad Institute found that the mutated version brings thicker hair shafts, more sweat glands, and less breast tissue. East Asian women are known for having comparatively small breasts and East Asians in general tend to have thick hair. The research team calculated that this gene originated in Southern China, which was warm and humid, meaning having more sweat glands would be advantageous to the hunter-gatherers who lived there. Geneticist Joshua Akey suggested that the mutant gene could also be favored by sexual selection in that the visible traits associated with this gene made the individual carrying it more attractive to potential mates. Yet a third explanation is offered by Kamberov, who argued that each of the traits due to the mutant gene could be favored at different times. Today, the mutant version of EDAR can be found among 93% of the Han Chinese, 70% among the Japanese and the Thai, and between 60% to 90% among the American Indians, who descended from East Asia.
The most recent Ice Age peaked in intensity between 19,000 and 25,000 years ago and ended about 12,000 years ago. As the glaciers that once covered Scandinavia all the way down to Northern France retreated, humans began returning to Northern Europe from the Southwest, modern-day Spain. But about 14,000 years ago, humans from Southeastern Europe, especially Greece and Turkey, began migrating to the rest of the continent, displacing the first group of humans. Analysis of genomic data revealed that all Europeans since 37,000 years ago have descended from a single founding population that survived the Ice Age, with specimens found in various parts of the continent, such as Belgium. Although this human population got displaced 33,000 years ago, a genetically related group began spreading across Europe 19,000 years ago. Recent divergence of Eurasian lineages was sped up significantly during the Last Glacial Maximum, the Mesolithic and the Neolithic, due to increased selection pressures and founder effects associated with migration. Alleles predictive of light skin have been found in Neanderthals, but the alleles for light skin in Europeans and East Asians, KITLG and ASIP, are (as of 2012) thought to have not been acquired by archaic admixture but recent mutations since the LGM. Phenotypes associated with the white or Caucasian populations of Western Eurasian stock emerge during the LGM, from about 19,000 years ago. The light skin pigmentation characteristic of modern Europeans is estimated to have spread across Europe in a "selective sweep" during the Mesolithic (5,000 years ago). The associated TYRP1 SLC24A5 and SLC45A2 alleles emerge around 19,000 years ago, still during the LGM, most likely in the Caucasus. Within the last 20,000 years or so, light skin has been favored by natural selection in East Asia, Europe, and North America. At the same time, Southern Africans tend to have lighter skin than their equatorial counterparts. In general, people living in higher latitudes tend to have lighter skin. The HERC2 variation for blue eyes first appears around 14,000 years ago in Italy and the Caucasus.
Inuit adaptation to high-fat diet and cold climate has been traced to a mutation dated the Last Glacial Maximum (20,000 years ago). Average cranial capacity among modern male human populations varies in the range of 1,200 to 1,450 cm3. Larger cranial volumes are associated with cooler climatic regions, with the largest averages being found in populations of Siberia and the Arctic. Humans living in Northern Asia and the Arctic have evolved the ability to develop thick layers of fat on their faces to keep warm. Moreover, the Inuit tend to have flat and broad faces, an adaptation that reduces the likelihood of frostbites. Both Neanderthal and Cro-Magnons had somewhat larger cranial volumes on average than modern Europeans, suggesting the relaxation of selection pressures for larger brain volume after the end of the LGM.
Australian Aboriginals living in the Central Desert, where the temperature can drop below freezing at night, have evolved the ability to reduce their core temperatures without shivering.
Early fossils of Homo sapiens suggest that members of this species had vastly different brains 300,000 years ago compared to today. In particular, they were elongated rather than globular in shape. Only fossils from 35,000 years ago or less share the same basic brain shape as that of current humans. Human brains appear to be shrinking over the last twenty thousand years. Modern human brains are about 10% smaller than those of the Cro-Magnons, who lived in Europe twenty to thirty thousand years ago. That is a difference comparable to a tennis ball. Scientists are not so sure about the implications of this finding. On one hand, it could be that humans are becoming less and less intelligent as their societies become ever more complex, which makes it easier for them to survive. On the other hand, shrinking brain sizes could be associated with lower levels of aggression. In any case, evidence for the shrinking human brain can be observed in Africa, China, and Europe.
Even though it has long been thought that human culture—broadly defined to be any learned behavior, including technology—has slowed down, if not halted, human evolution, biologists working in the early twenty-first century A.D. have come to the conclusion that instead, human culture itself is a force of selection. Scans of the entire human genome suggests large parts of it is under active selection within the last 10,000 to 20,000 years or so, which is recent in evolutionary terms. Although the details of such genes remain unclear (as of 2010), they can still be categorized for likely functionality according to the structures of the proteins for which they code. Many such genes are linked to the immune system, the skin, metabolism, digestion, bone development, hair growth, smell and taste, and brain function. Since the culture of behaviorally modern humans undergoes rapid change, it is possible that human culture has accelerated human evolution within the last 50,000 years or so. While this possibility remains unproven, mathematical models do suggest that gene-culture interactions can give rise to especially speedy biological evolution. If this is true, then humans are evolving to adapt to the selective pressures they created themselves.
Holocene (12,000 years ago till present)
Neolithic or New Stone Age
Blue eyes are an adaptation for living in regions where the amounts of light are limited because they allow more light to come in than brown eyes. A research program by geneticist Hans Eiberg and his team at the University of Copenhagen from the 1990s to 2000s investigating the origins of blue eyes revealed that a mutation in the gene OCA2 is responsible for this trait. According to them, all humans initially had brown eyes and the OCA2 mutation took place between 6,000 and 10,000 years ago. It dilutes the production of melanin, responsible for the pigmentation of human hair, eye, and skin color. The mutation does not completely switch off melanin production, however, as that would leave the individual with a condition known as albinism. Variations in eye color from brown to green can be explained via the variation in the amounts of melanin produced in the iris. While brown-eyed individuals share a large area in their DNA controlling melanin production, blue-eyed individuals have only a small region. By examining mitochondrial DNA of people from multiple countries, Eiberg and his team concluded blue-eyed individuals all share a common ancestor.
In 2018, an international team of researchers from Israel and the United States announced their genetic analysis of 6,500-year-old excavated human remains in Israel's Upper Galilee region revealed a number of traits not found in the humans who had previously inhabited the area, including blue eyes. They concluded that the region experienced a significant demographic shift 6,000 years ago due to migration from Anatolia and the Zagros mountains (in modern-day Turkey and Iran) and that this change contributed to the development of the Chalcolithic culture in the region.
In 2006, population geneticist Jonathan Pritchard and his colleagues studied the populations of Africa, East Asia, and Europe and identified some 700 regions of the human genome as having been shaped by natural selection between 15,000 and 5,000 years ago. These genes affect the senses of smell and taste, skin color, digestion, bone structure, and brain function. According to Spencer Wells, director of the Genographic Project of the National Geographic Society, such a study helps anthropologists explain in detail why peoples from different parts of the globe can be so strikingly different in appearance even though most of their DNA is identical.
The advent of agriculture has played a key role in the evolutionary history of humanity. Early farming communities benefited from new and comparatively stable sources of food, but were also exposed to new and initially devastating diseases such as measles and smallpox. Eventually, genetic resistance to such diseases evolved and humans living today are descendants of those who survived the agricultural revolution and reproduced. Diseases are one of the strongest forces of evolution acting on Homo sapiens. As this species migrated throughout Africa and began colonizing new lands outside the continent around 100,000 years ago, they came into contact with and helped spread a variety of pathogens with deadly consequences. In addition, the dawn of agriculture led to the rise of major disease outbreaks. Malaria is the oldest known of human contagions, traced to West Africa around 100,000 years ago, before humans began migrating out of the continent. Malarial infections surged around 10,000 years ago, raising the selective pressures upon the affected populations, leading to the evolution of resistance.
A study by anthropologists John Hawks, Henry Harpending, Gregory Cochran, and colleagues suggests that human evolution has sped up significantly since the beginning of the Holocene, at an estimated pace of around 100 times faster than during the Paleolithic, primarily in the farming populations of Eurasia. Thus, humans living in the twenty-first century are more different from their ancestors of 5,000 years ago than their ancestors from that era were to the Neanderthals who went extinct around 30,000 years ago. They tied this effect to new selection pressures arising from new diets, new modes of habitation, and immunological pressures related to the domestication of animals. For example, populations that cultivate rice, wheat, and other grains have gained the ability to digest starch thanks to an enzyme called amylase, found in saliva. In addition, having a larger population means having more mutations, the raw material on which natural selection acts.
Hawks and colleagues scanned data from the International HapMap Project of Africans, Asians, and Europeans for SNPs and found evidence of evolution speeding up in 1800 genes, or 7% of the human genome. They also discovered that human populations in Africa, Asia, and Europe were evolving along divergent paths, becoming ever more different, and that there was very little gene flow among them. Most of the new traits are unique to their continent of origin.
Humans living in humid tropical areas show the least sign of evolution, meaning ancestral humans were especially well-suited to these places. Only when humans migrated out of them did natural selective pressures arise. Moreover, African populations have the highest amounts of genetic diversity; the further one moves from Africa, the more homogeneous people become genetically. In fact, most of the variation in the human genome is due not to natural selection but rather neutral mutations and random shuffling of genes down the generations.
John Hawks reported evidence of recent evolution in the human brain within the last 5,000 years or so. Measurements of the skull suggests that the human brain has shrunk by about 150 cubic centimeters, or roughly ten percent. This is likely due to the growing specialization in modern societies centered around agriculture rather than hunting and gathering. More broadly, human brain sizes have been diminishing since at least 100,000 years ago, though the change was most significant within the last 12,000 years. 100,000 years ago, the average brain size was about 1,500 cubic centimeters, compared to around 1,450 cubic centimeters 12,000 years ago and 1,350 today.
Examples for adaptations related to agriculture and animal domestication include East Asian types of ADH1B associated with rice domestication, and lactase persistence.
About ten thousand years ago, the rice-cultivating residents of Southern China discovered that they could make alcoholic beverages by fermentation. Drunkenness likely became a serious threat to survival and a mutant gene for an enzyme that decomposes alcohol into something safe and makes people's faces turn red, alcohol dehydrogenase, gradually spread throughout the rest of China.
Around 11,000 years ago, as agriculture was replacing hunting and gathering in the Middle East, people invented ways to reduce the concentrations of lactose in milk by fermenting it to make yogurt and cheese. People lost the ability to digest lactose as they matured and as such lost the ability to consume milk. Thousands of years later, a genetic mutation enabled people living in Europe at the time to continue producing lactase, an enzyme that digests lactose, throughout their lives, allowing them to drink milk after weaning and survive bad harvests.
These two key developments paved the way for communities of farmers and herders to rapidly displace the hunter-gatherers who once prevailed across Europe. Today, lactase persistence can be found in 90% or more of the populations in Northwestern and Northern Central Europe, and in pockets of Western and Southeastern Africa, Saudi Arabia, and South Asia. It is not as common in Southern Europe (40%) because Neolithic farmers had already settled there before the mutation existed. On the other hand, it is rather rare in inland Southeast Asia and Southern Africa. While all Europeans with lactase persistence share a common ancestor for this ability, pockets of lactase persistence outside Europe are likely due to separate mutations. The European mutation, called the LP allele, is traced to modern-day Hungary, 7,500 years ago. In the twenty-first century, about 35% of the human population is capable of digesting lactose after the age of seven or eight. Milk-drinking humans could produce offspring up to 19% more fertile than those without the ability, putting the mutation among those under the strongest selection known. As an example of gene-culture co-evolution, communities with lactase persistence and dairy farming took over Europe in several hundred generations, or thousands of years. This raises a chicken-and-egg type of question: which came first, dairy farming or lactase persistence? To answer this question, population geneticists examined DNA samples extracted from skeletons found in archeological sites in Germany, Hungary, Poland, and Lithuania dating from between 3,800 and 6,000 years ago. They did not find any evidence of the LP allele. Hence, Europeans began dairy farming before they gained the ability to drink milk after early childhood.
A Finnish research team reported that the European mutation that allows for lactase persistence is not found among the milk-drinking and dairy-farming Africans, however. Sarah Tishkoff and her students confirmed this by analyzing DNA samples from Tanzania, Kenya, and Sudan, where lactase persistence evolved independently. The uniformity of the mutations surrounding the lactase gene suggests that lactase persistence spread rapidly throughout this part of Africa. According to Tishkoff's data, this mutation first appeared between 3,000 and 7,000 years ago, and has been strongly favored by natural selection, more strongly than even resistance to malaria, in fact. In this part of the world, it provides some protection against drought and enables people to drink milk without diarrhea, which causes dehydration.
Lactase persistence is a rare ability among mammals. It is also a clear and simple example of convergent evolution in humans because it involves a single gene. Other examples of convergent evolution, such as the light skin of Europeans and East Asians or the various means of resistance to malaria, are much more complicated.
The shift towards settled communities based on farming was a significant cultural change, which in turn may have accelerated human evolution. Agriculture brought about an abundance of cereals, enabling women to wean their babies earlier and have more children over shorter periods of time. Despite the vulnerability of densely populated communities to diseases, this led to a population explosion and thus more genetic variation, the raw material on which natural selection acts. Diets in early agricultural communities were deficient in many nutrients, including vitamin D. This could be one reason why natural selection has favored fair skin among Europeans, as it increases UV absorption and synthesis of vitamin D.
Paleoanthropologist Richard G. Klein of Stanford University told the New York Times that while it was difficult to correlate a given genetic change with a specific archeological period, it was possible to identify a number of modifications as due to the rise of agriculture. Rice cultivation spread across China between 7,000 and 6,000 years ago and reached Europe at about the same time. Scientists have had trouble finding Chinese skeletons before that period resembling that of a modern Chinese person or European skeletons older than 10,000 years similar to that of a modern European.
Among the list of genes Jonathan Pritchard and his team studied were five that influenced complexion. Selected versions of the genes, thought to have first emerged 6,600 years ago, were found only among Europeans and were responsible for their pale skin. The consensus among anthropologists is that when the first anatomically modern humans arrived in Europe 45,000 years ago, they shared the dark skin of their African ancestors but eventually acquired lighter skin as an adaptation that helped them synthesize vitamin D using sunlight. This means that either the Europeans acquired their light skin much more recently or that this was a continuation of an earlier trend. Because East Asians are also pale, nature achieved the same result either by selecting different genes not detected by the test or by doing so to the same genes but thousands of years earlier, making such changes invisible to the test.
Non-human primates have no pigments in their skin because they have fur. But when humans lost their fur—enabling them to sweat efficiently—they needed dark skin to protect themselves against ultraviolet radiation. Later research revealed that the so-called golden gene, thus named because of the color it gives to zebrafish, is ubiquitous among Europeans but rare among East Asians, suggesting there was little gene flow between the two populations. Among East Asians, a different gene, DCT, likely contributed to their fair skin.
Bronze Age to Medieval Era
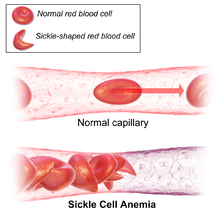
Resistance to malaria is a well-known example of recent human evolution. This disease attacks humans early in life. Thus humans who are resistant enjoy a higher chance of surviving and reproducing. While humans have evolved multiple defenses against malaria, sickle cell anemia—a condition in which red blood cells are deformed into sickle shapes, thereby restricting blood flow—is perhaps the best known. Sickle cell anemia makes it more difficult for the malarial parasite to infect red blood cells. This mechanism of defense against malaria emerged independently in Africa and in Pakistan and India. Within 4,000 years it has spread to 10-15% of the populations of these places. Another mutation that enabled humans to resist malaria that is strongly favored by natural selection and has spread rapidly in Africa is the inability of synthesize the enzyme glucose-6-phosphate dehydrogenase, or G6PD.
A combination of poor sanitation and high population densities proved ideal for the spread of contagious diseases which was deadly for the residents of ancient cities. Evolutionary thinking would suggest that people living in places with long-standing urbanization dating back millennia would have evolved resistance to certain diseases, such as tuberculosis and leprosy. Using DNA analysis and archeological findings, scientists from the University College London and the Royal Holloway studied samples from 17 sites in Europe, Asia, and Africa. They learned that, indeed, long-term exposure to pathogens has led to resistance spreading across urban populations. Urbanization is therefore a selective force that has influenced human evolution. The allele in question is named SLC11A1 1729+55del4. Scientists found that among the residents of places that have been settled for thousands of years, such as Susa in Iran, this allele is ubiquitous whereas in places with just a few centuries of urbanization, such as Yakutsk in Siberia, only 70-80% of the population have it.
Adaptations have also been found in modern populations living in extreme climatic conditions such as the Arctic, as well as immunological adaptations such as resistance against brain disease in populations practicing mortuary cannibalism, or the consumption of human corpses. Inuit have the ability to thrive on the lipid-rich diets consisting of Arctic mammals. Human populations living in regions of high attitudes, such as the Tibetan Plateau, Ethiopia, and the Andes benefit from a mutation that enhances the concentration of oxygen in their blood. This is achieved by having more capillaries, increasing their capacity for carrying oxygen. This mutation is believed to be around 3,000 years old.
Geneticist Ryosuke Kimura and his team at the Tokai University School of Medicine discovered that an allele called EDAR, practically absent among Europeans and Africans but common among East Asians, gives rise to thicker hair, presumably as an adaptation to the cold. Kohichiro Yoshihura and his team at Nagasaki University found that a variant of the gene ABCC11 produces dry ear wax among East Asians. Africans and Europeans by contrast share the older version of the gene, producing wet ear wax. However, it is not known what evolutionary advantage, if any, wet ear wax confers, so this variant was likely selected for some other trait, such as making people sweat less. What scientists do know, however, is that dry ear wax is strongly favored by natural selection in East Asia.
A recent adaptation has been proposed for the Austronesian Sama-Bajau, also known as the Sea Gypsies or Sea Nomads, developed under selection pressures associated with subsisting on free-diving over the past thousand years or so. As maritime hunter-gatherers, the ability to dive for long periods of times plays a crucial role in their survival. Due to the mammalian dive reflex, the spleen contracts when the mammal dives and releases oxygen-carrying red blood cells. Over time, individuals with larger spleens were more likely to survive and thrive because free-diving can actually be quite dangerous. By contrast, communities centered around farming show no signs of evolving to have larger spleens. Because the Sama-Bajau show no interest in abandoning this lifestyle, there is no reason to believe further adaptation will not occur.
Advances in the biology of genomes have enabled geneticists to investigate the course of human evolution within centuries or even decades. Jonathan Pritchard and a postdoctoral fellow, Yair Field, found a way to track changes in the frequency of an allele using huge genomic data sets. They did this by counting the singletons, or changes of single DNA bases, which are likely to be recent because they are rare and have not spread throughout the population. Since alleles bring neighboring DNA regions with them as they move around the genome, the number of singletons can be used to roughly estimate how quickly the allele has changed its frequency. This approach can unveil evolution within the last 2,000 years or a hundred human generations. Armed with this technique and data from the UK10K project, Pritchard and his team found that alleles for lactase persistence, blond hair, and blue eyes have spread rapidly among Britons within the last two millennia or so. Britain's cloudy skies may have played a role in that the genes for fair hair could also bring fair skin, reducing the chances of vitamin D deficiency. Sexual selection could play a role, too, driven by fondness of mates with blond hair and blue eyes. The technique also enabled them to track the selection of polygenic traits—those affected by a multitude of genes, rather than just one—such as height, infant head circumferences, and female hip sizes (crucial for giving birth). They found that natural selection has been favoring increased height and larger head and female hip sizes among Britons. Moreover, lactase persistence showed signs of active selection during the same period. However, evidence for the selection of polygenic traits is weaker than those affected only by one gene.
A 2012 paper studied the DNA sequence of around 6,500 Americans of European and African descent and confirmed earlier work indicating that the majority of changes to a single letter in the sequence (single nucleotide variants) were accumulated within the last 5,000-10,000 years. Almost three quarters arose in the last 5,000 years or so. About 14% of the variants are potentially harmful, and among those, 86% were 5,000 years old or younger. The researchers also found that European Americans had accumulated a much larger number of mutations than African Americans. This is likely a consequence of their ancestors' migration out of Africa, which resulted in a genetic bottleneck; there were few mates available. Despite the subsequent exponential growth in population, natural selection has not had enough time to eradicate the harmful mutations. While humans today carry far more mutations than their ancestors did 5,000 years ago, they are not necessarily more vulnerable to illnesses because these might be caused by multiple mutations. It does, however, confirm earlier research suggesting that common diseases are not caused by common gene variants. In any case, the fact that the human gene pool has accumulated so many mutations over such a short period of time—in evolutionary terms—and that the human population has exploded in that time mean that humanity is more evolvable than ever before. Natural selection might eventually catch up with the variations in the gene pool, as theoretical models suggest that evolutionary pressures increase as a function of population size.
Industrial Revolution to present
Geneticist Steve Jones told the BBC that during the sixteenth century, only a third of English babies survived till the age of 21, compared to 99% in the twenty-first century. Medical advances, especially those made in the twentieth century, made this change possible. Yet while people from the developed world today are living longer and healthier lives, many are choosing to have just a few or no children at all, meaning evolutionary forces continue to act on the human gene pool, just in a different way.
While modern medicine appears to shield humanity from the pressures of natural selection, it does not prevent other evolutionary processes from taking place. According to neutral selection theory, natural selection affects only 8% of the human genome, meaning mutations in the remaining parts of the genome can change their frequency by pure chance. If natural selective pressures are reduced, then traits that are normally purged are not removed as quickly, which could increase their frequency and speed up evolution. There is evidence that the rate of human mutation is rising. For humans, the largest source of heritable mutations is sperm; a man accumulates more and more mutations in his sperm as he ages. Hence, men delaying reproduction can affect human evolution. The accumulation of so many mutations in a short period of time could pose genetic problems for future human generations.
A 2012 study led by Augustin Kong suggests that the number of de novo (or new) mutations increases by about two per year of delayed reproduction by the father and that the total number of paternal mutations doubles every 16.5 years.
Dependence on modern medicine itself is another evolutionary time bomb. For a long time, it has reduced the fatality of genetic defects and contagious diseases, allowing more and more humans to survive and reproduce, but it has also enabled maladaptive traits that would otherwise be culled to accumulate in the gene pool. This is not a problem as long as access to modern healthcare is maintained. But natural selective pressures will mount considerably if that is taken away. Nevertheless, dependence on medicine rather than genetic adaptations will likely be the driving force behind humanity's fight against diseases for the foreseeable future. Moreover, while the introduction of antibiotics initially reduced the mortality rates due to infectious diseases by significant amounts, abuse has led to the rise of resistant strains of bacteria, making many illnesses major causes of death once again.
Human jaws and teeth have been shrinking in proportion with the decrease in body size in the last 30,000 years as a result of new diets and technology. There are many individuals today who do not have enough space in their mouths for their third molars (or wisdom teeth) due to reduced jaw sizes. In the twentieth century, the trend toward smaller teeth appeared to have been slightly reversed due to the introduction of fluoride, which thickens dental enamel, thereby enlarging the teeth.
In the middle of the eighteenth century, the average height of Dutch soldiers was 165 cm, well below European and American averages. However, 150 years later, the Dutch gained an average of 20 cm while the Americans only 6 cm. This is due to the fact that tall Dutchmen on average had more children than those who were short, as Dutchwomen found them more attractive, and that while tall Dutchwomen on average had fewer children than those of medium heights, they did have more children than those who were short. Things like good nutrition and good healthcare did not play as important a role as biological evolution. By contrast, in some other countries such as the United States, for example, men of average height and short women tended to have more children.
Recent research suggests that menopause is evolving to occur later. Other reported trends appear to include lengthening of the human reproductive period and reduction in cholesterol levels, blood glucose and blood pressure in some populations.
Population geneticist Emmanuel Milot and his team studied recent human evolution in an isolated Canadian island using 140 years of church records. They found that selection favored younger age at first birth among women. In particular, the average age at first birth of women from Coudres Island (Île aux Coudres), 80 km northeast of Québec City, decreased by four years between 1800 and 1930. Women who started having children sooner generally ended up with more children in total who survive till adulthood. In other words, for these French-Canadian women, reproductive success was associated with lower age at first childbirth. Maternal age at first birth is a highly heritable trait.
Human evolution continues during the modern era, including among industrialized nations. Things like access to contraception and the freedom from predators do not stop natural selection. Among developed countries, where life expectancy is high and infant mortality rates are low, selective pressures are the strongest on traits that influence the number of children a human has. It is speculated that alleles influencing sexual behavior would be subject to strong selection, though the details of how genes can affect said behavior remain unclear.
Historically, as a by-product of the ability to walk upright, humans evolved to have narrower hips and birth canals and to have larger heads. Compared to other close relatives such as chimpanzees, childbirth is a highly challenging and potentially fatal experience for humans. Thus began an evolutionary tug-of-war. For babies, having larger heads proved beneficial as long as their mothers' hips were wide enough. If not, both mother and child typically died. This is an example of balancing selection, or the removal of extreme traits. In this case, heads that were too large or small were selected against. This evolutionary tug-of-war attained an equilibrium, making these traits remain more or less constant over time while allowing for genetic variation to flourish, thus paving the way for rapid evolution should selective forces shift their direction.
All this changed in the twentieth century as Cesarean sections (or C-sections) became safer and more common in some parts of the world. Larger head sizes continue to be favored while selective pressures against smaller hip sizes have diminished. Projecting forward, this means that human heads would continue to grow while hip sizes would not. As a result of increasing fetopelvic disproportion, C-sections would become more and more common in a positive feedback loop, though not necessarily to the extent that natural childbirth would become obsolete.
Paleoanthropologist Briana Pobiner of the Smithsonian Institute noted that cultural factors could play a role in the widely different rates of C-sections across the developed and developing worlds. Daghni Rajasingam of the Royal College of Obstetricians observed that the increasing rates of diabetes and obesity among women of reproductive age also boost the demand for C-sections. Biologist Philipp Mitteroecker from the University of Vienna and his team estimated that about six percent of all births worldwide were obstructed and required medical intervention. In the United Kingdom, one quarter of all births involved the C-section while in the United States, the number was one in three. Mitteroecker and colleagues discovered that the rate of C-sections has gone up 10% to 20% since the mid-twentieth century. They argued that because the availability of safe Cesarean sections significantly reduced maternal and infant mortality rates in the developed world, they have induced an evolutionary change. However, "It's not easy to foresee what this will mean for the future of humans and birth," Mitteroecker told The Independent. This is because the increase in baby sizes is limited by the mother's metabolic capacity and modern medicine, which makes it more likely that neonates who are born prematurely or are underweight to survive.
Researchers participating in the Framingham Heart Study, which began in 1948 and was intended to investigate the cause of heart disease among women and their descendants in Framingham, Massachusetts, found evidence for selective pressures against high blood pressure due to the modern Western diet, which contains high amounts of salt, known for raising blood pressure. They also found evidence for selection against hypercholesterolemnia, or high levels of cholesterol in the blood. Evolutionary geneticist Stephen Stearns and his colleagues reported signs that women were gradually becoming shorter and heavier. Stearns argued that human culture and changes humans have made on their natural environments are driving human evolution rather than putting the process to a halt. The data indicates that the women were not eating more; rather, the ones who were heavier tended to have more children. Stearns and his team also discovered that the subjects of the study tended to reach menopause later; they estimated that if the environment remains the same, the average age at menopause will increase by about a year in 200 years, or about ten generations. All these traits have medium to high heritability. Given the starting date of the study, the spread of these adaptations can be observed in just a few generations.
By analyzing genomic data of 60,000 individuals of Caucasian descent from Kaiser Permanente in Northern California, and 150,000 people the UK Biobank, evolutionary geneticist Joseph Pickrell and evolutionary biologist Molly Przeworski were able to identify signs of biological evolution among living human generations. For the purposes of studying evolution, one lifetime is the shortest possible time scale. An allele associated with difficulty withdrawing from tobacco smoking dropped in frequency among the British but not among the Northern Californians. This suggests that heavy smokers—who were common in Britain during the 1950s but not in Northern California—were selected against. A set of alleles linked to later menarche was more common among women who lived for longer. An allele called ApoE4, linked to Alzheimer's disease, fell in frequency as carriers tended to not live for very long. In fact, these were the only traits that reduced life expectancy Pickrell and Przeworski found, which suggests that other harmful traits probably have already been eradicated. Only among older people are the effects of Alzheimer's disease and smoking visible. Moreover, smoking is a relatively recent trend. It is not entirely clear why such traits bring evolutionary disadvantages, however, since older people have already had children. Scientists proposed that either they also bring about harmful effects in youth or that they reduce an individual's inclusive fitness, or the tendency of organisms that share the same genes to help each other. Thus, mutations that make it difficult for grandparents to help raise their grandchildren are unlikely to propagate throughout the population. Pickrell and Przeworski also investigated 42 traits determined by multiple alleles rather than just one, such as the timing of puberty. They found that later puberty and older age of first birth were correlated with higher life expectancy.
Larger sample sizes allow for the study of rarer mutations. Pickrell and Przeworski told The Atlantic that a sample of half a million individuals would enable them to study mutations that occur among only 2% of the population, which would provide finer details of recent human evolution. While studies of short time scales such as these are vulnerable to random statistical fluctuations, they can improve understanding of the factors that affect survival and reproduction among contemporary human populations.
Evolutionary geneticist Jaleal Sanjak and his team analyzed genetic and medical information from more than 200,000 women over the age of 45 and 150,000 men over the age of 50—people who have passed their reproductive years—from the UK Biobank and identified 13 traits among women and ten among men that were linked to having children at a younger age, having a higher body-mass index, fewer years of education, and lower levels of fluid intelligence, or the capacity for logical reasoning and problem solving. Sanjak noted, however, that it was not known whether having children actually made women heavier or being heavier made it easier to reproduce. Because taller men and shorter women tended to have more children and because the genes associated with height affect men and women equally, the average height of the population will likely remain the same. Among women who had children later, those with higher levels of education had more children.
Evolutionary biologist Hakhamanesh Mostafavi led a 2017 study that analyzed data of 215,000 individuals from just a few generations the United Kingdom and the United States and found a number of genetic changes that affect longevity. The ApoE allele linked to Alzheimer's disease was rare among women aged 70 and over while the frequency of the CHRNA3 gene associated with smoking addiction among men fell among middle-aged men and up. Because this is not itself evidence of evolution, since natural selection only cares about successful reproduction not longevity, scientists have proposed a number of explanations. Men who live longer tend to have more children. Men and women who survive till old age can help take care of both their children and grandchildren, in benefits their descendants down the generations. This explanation is known as the grandmother hypothesis. It is also possible that Alzheimer's disease and smoking addiction are also harmful earlier in life, but the effects are more subtle and larger sample sizes are required in order to study them. Mostafavi and his team also found that mutations causing health problems such as asthma, having a high body-mass index and high cholesterol levels were more common among those with shorter lifespans while mutations those leading to delayed puberty and reproduction were more common among long living individuals. According to geneticist Jonathan Pritchard, while the link between fertility and longevity was identified in previous studies, those did not entirely rule out the effects of educational and financial status—people who rank high in both tend to have children later in life; this seems to suggest the existence of an evolutionary trade-off between longevity and fertility.
In South Africa, where large numbers of people are infected with HIV, some have genes that help them combat this virus, making it more likely that they would survive and pass this trait onto their children. If the virus persists, humans living in this part of the world could become resistant to it in as little as hundreds of years. However, because HIV evolves more quickly than humans, it will more likely be dealt with technologically rather than genetically.
A 2017 study by researchers from Northwestern University unveiled a mutation among the Old Order Amish living in Berne, Indiana, that suppressed their chances of having diabetes and extends their life expectancy by about ten years on average. That mutation occurred in the gene called Serpine1, which codes for the production of the protein PAI-1 (plasminogen activator inhibitor), which regulates blood clotting and plays a role in the aging process. About 24% of the people sampled carried this mutation and had a life expectancy of 85, higher than the community average of 75. Researchers also found the telomeres—non-functional ends of human chromosomes—of those with the mutation to be longer than those without. Because telomeres shorten as the person ages, they determine the person's life expectancy. Those with longer telomeres tend to live longer. At present, the Amish live in 22 U.S. states plus the Canadian province of Ontario. They live simple lifestyles that date back centuries and generally insulate themselves from modern North American society. They are mostly indifferent towards modern medicine, but scientists do have a healthy relationship with the Amish community in Berne. Their detailed genealogical records make them ideal subjects for research.
Multidisciplinary research suggests that ongoing evolution could help explain the rise of certain medical conditions such as autism and autoimmune disorders. Autism and schizophrenia may be due to genes inherited from the mother and the father which are over-expressed and which fight a tug-of-war in the child's body. Allergies, asthma, and autoimmune disorders appear linked to higher standards of sanitation, which prevent the immune systems of modern humans from being exposed to various parasites and pathogens the way their ancestors' were, making them hypersensitive and more likely to overreact. The human body is not built from a professionally engineered blue print but a system shaped over long periods of time by evolution with all kinds of trade-offs and imperfections. Understanding the evolution of the human body can help medical doctors better understand and treat various disorders. Research in evolutionary medicine suggests that diseases are prevalent because natural selection favors reproduction over health and longevity. In addition, biological evolution is slower than cultural evolution and humans evolve more slowly than pathogens.
Whereas in the ancestral past, humans lived in geographically isolated communities where inbreeding was rather common,
modern transportation technologies have made it much easier for people
to travel great distances and facilitated further genetic mixing, giving
rise to additional variations in the human gene pool. It also enables the spread of diseases worldwide, which can have an effect on human evolution. Besides the selection and flow of genes and alleles, another mechanism of biological evolution is epigenetics,
or changes not to the DNA sequence itself, but rather the way it is
expressed. Scientists already know that chronic illnesses and stress are
epigenetic mechanisms