 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɒksɪˈtoʊsɪn/ |
| Physiological data | |
| Source tissues | pituitary gland |
| Target tissues | wide spread |
| Receptors | oxytocin receptor |
| Antagonists | atosiban |
| Precursor | oxytocin/neurophysin I prepropeptide |
| Metabolism | liver and other oxytocinases |
| Pharmacokinetic data | |
| Protein binding | 30% |
| Metabolism | liver and other oxytocinases |
| Elimination half-life | 1–6 min (IV) ~2 h (intranasal) |
| Excretion | Biliary and kidney |
| Identifiers | |
| CAS Number | |
|---|---|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.045 |
| Chemical and physical data | |
| Formula | C43H66N12O12S2 |
| Molar mass | 1007.19 g·mol−1 |
| 3D model (JSmol) | |
Oxytocin (Oxt) is a peptide hormone and neuropeptide. It is normally produced in the hypothalamus and released by the posterior pituitary. It plays a role in social bonding, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to love and in labor. This helps with birth, bonding with the baby, and milk production.
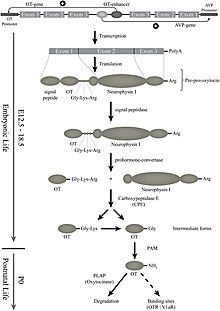
Oxytocin is derived by enzymatic splitting from the peptide precursor encoded by the human OXT gene. The deduced structure of the active nonapeptide is:
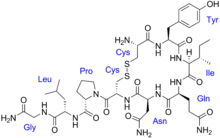
·Cys – Tyr – Ile – Gln – Asn – Cys – Pro – Leu – Gly – NH2, or CYIQNCPLG-NH2.
Discovery
Oxytocin was discovered by Henry Dale in 1906. Its molecular structure was determined in 1952. It is also used as a medication to facilitate childbirth (see oxytocin (medication) for more information). In the early 1950s, American biochemist Vincent du Vigneaud found that oxytocin is made up of nine amino acids, and he identified its amino acid sequence. In 1953 du Vigneaud carried out the synthesis of oxytocin, making it the first polypeptide hormone to be synthesized.
Biochemistry
Estrogen has been found to increase the secretion of oxytocin and to increase the expression of its receptor, the oxytocin receptor, in the brain. In women, a single dose of estradiol has been found to be sufficient to increase circulating oxytocin concentrations.
Biosynthesis
The oxytocin peptide is synthesized as an inactive precursor protein from the OXT gene. This precursor protein also includes the oxytocin carrier protein neurophysin I. The inactive precursor protein is progressively hydrolyzed into smaller fragments (one of which is neurophysin I) via a series of enzymes. The last hydrolysis that releases the active oxytocin nonapeptide is catalyzed by peptidylglycine alpha-amidating monooxygenase (PAM).
The activity of the PAM enzyme system is dependent upon vitamin C (ascorbate), which is a necessary vitamin cofactor. By chance, sodium ascorbate by itself was found to stimulate the production of oxytocin from ovarian tissue over a range of concentrations in a dose-dependent manner. Many of the same tissues (e.g. ovaries, testes, eyes, adrenals, placenta, thymus, pancreas) where PAM (and oxytocin by default) is found are also known to store higher concentrations of vitamin C.
Oxytocin is known to be metabolized by the oxytocinase, leucyl/cystinyl aminopeptidase. Other oxytocinases are also known to exist. Amastatin, bestatin (ubenimex), leupeptin, and puromycin have been found to inhibit the enzymatic degradation of oxytocin, though they also inhibit the degradation of various other peptides, such as vasopressin, met-enkephalin, and dynorphin A.
Neural sources
In the hypothalamus, oxytocin is made in magnocellular neurosecretory cells of the supraoptic and paraventricular nuclei, and is stored in Herring bodies at the axon terminals in the posterior pituitary. It is then released into the blood from the posterior lobe (neurohypophysis) of the pituitary gland. These axons (likely, but dendrites have not been ruled out) have collaterals that innervate neurons in the nucleus accumbens, a brain structure where oxytocin receptors are expressed. The endocrine effects of hormonal oxytocin and the cognitive or behavioral effects of oxytocin neuropeptides are thought to be coordinated through its common release through these collaterals. Oxytocin is also produced by some neurons in the paraventricular nucleus that project to other parts of the brain and to the spinal cord. Depending on the species, oxytocin receptor-expressing cells are located in other areas, including the amygdala and bed nucleus of the stria terminalis.
In the pituitary gland, oxytocin is packaged in large, dense-core vesicles, where it is bound to neurophysin I as shown in the inset of the figure; neurophysin is a large peptide fragment of the larger precursor protein molecule from which oxytocin is derived by enzymatic cleavage.
Secretion of oxytocin from the neurosecretory nerve endings is regulated by the electrical activity of the oxytocin cells in the hypothalamus. These cells generate action potentials that propagate down axons to the nerve endings in the pituitary; the endings contain large numbers of oxytocin-containing vesicles, which are released by exocytosis when the nerve terminals are depolarised.
Non-neural sources
Endogenous oxytocin concentrations in the brain have been found to be as much as 1000-fold higher than peripheral levels.
Outside the brain, oxytocin-containing cells have been identified in several diverse tissues, including in females in the corpus luteum and the placenta; in males in the testicles' interstitial cells of Leydig; and in both sexes in the retina, the adrenal medulla, the thymus and the pancreas. The finding of significant amounts of this classically "neurohypophysial" hormone outside the central nervous system raises many questions regarding its possible importance in these diverse tissues.
Male
The Leydig cells in some species have been shown to possess the biosynthetic machinery to manufacture testicular oxytocin de novo, to be specific, in rats (which can synthesize vitamin C endogenously), and in guinea pigs, which, like humans, require an exogenous source of vitamin C (ascorbate) in their diets.
Female
Oxytocin is synthesized by corpora lutea of several species, including ruminants and primates. Along with estrogen, it is involved in inducing the endometrial synthesis of prostaglandin F2α to cause regression of the corpus luteum.
Evolution
Virtually all vertebrates have an oxytocin-like nonapeptide hormone that supports reproductive functions and a vasopressin-like nonapeptide hormone involved in water regulation. The two genes are usually located close to each other (less than 15,000 bases apart) on the same chromosome, and are transcribed in opposite directions (however, in fugu, the homologs are further apart and transcribed in the same direction).
The two genes are believed to result from a gene duplication event; the ancestral gene is estimated to be about 500 million years old and is found in cyclostomata (modern members of the Agnatha).
Biological function
Oxytocin has peripheral (hormonal) actions, and also has actions in the brain. Its actions are mediated by specific oxytocin receptors. The oxytocin receptor is a G-protein-coupled receptor, OT-R, which requires magnesium and cholesterol and is expressed in myometrial cells. It belongs to the rhodopsin-type (class I) group of G-protein-coupled receptors.
Studies have looked at oxytocin's role in various behaviors, including orgasm, social recognition, pair bonding, anxiety, in-group bias, situational lack of honesty, autism, and maternal behaviors.
Physiological
The peripheral actions of oxytocin mainly reflect secretion from the pituitary gland. The behavioral effects of oxytocin are thought to reflect release from centrally projecting oxytocin neurons, different from those that project to the pituitary gland, or that are collaterals from them. Oxytocin receptors are expressed by neurons in many parts of the brain and spinal cord, including the amygdala, ventromedial hypothalamus, septum, nucleus accumbens, and brainstem, although the distribution differs markedly between species. Furthermore, the distribution of these receptors changes during development and has been observed to change after parturition in the montane vole.
- Milk ejection reflex/Letdown reflex: in lactating (breastfeeding) mothers, oxytocin acts at the mammary glands, causing milk to be 'let down' into lactiferous ducts, from where it can be excreted via the nipple. Suckling by the infant at the nipple is relayed by spinal nerves to the hypothalamus. The stimulation causes neurons that make oxytocin to fire action potentials in intermittent bursts; these bursts result in the secretion of pulses of oxytocin from the neurosecretory nerve terminals of the pituitary gland.
- Uterine contraction: important for cervical dilation before birth, oxytocin causes contractions during the second and third stages of labor. Oxytocin release during breastfeeding causes mild but often painful contractions during the first few weeks of lactation. This also serves to assist the uterus in clotting the placental attachment point postpartum. However, in knockout mice lacking the oxytocin receptor, reproductive behavior and parturition are normal.
- In male rats, oxytocin may induce erections. A burst of oxytocin is released during ejaculation in several species, including human males; its suggested function is to stimulate contractions of the reproductive tract, aiding sperm release.
- Human sexual response: Oxytocin levels in plasma rise during sexual stimulation and orgasm. At least two uncontrolled studies have found increases in plasma oxytocin at orgasm – in both men and women. Plasma oxytocin levels are increased around the time of self-stimulated orgasm and are still higher than baseline when measured five minutes after self arousal. The authors of one of these studies speculated that oxytocin's effects on muscle contractibility may facilitate sperm and egg transport.
- In a study measuring oxytocin serum levels in women before and after sexual stimulation, the author suggests it serves an important role in sexual arousal. This study found genital tract stimulation resulted in increased oxytocin immediately after orgasm. Another study reported increases of oxytocin during sexual arousal could be in response to nipple/areola, genital, and/or genital tract stimulation as confirmed in other mammals. Murphy et al. (1987), studying men, found that plasma oxytocin levels remain unchanged during sexual arousal, but that levels increase sharply after ejaculation, returning to baseline levels within 30 minutes. In contrast, vasopressin was increased during arousal but returned to baseline at the time of ejaculation. The study concludes that (in males) vasopressin is secreted during arousal, while oxytocin is only secreted after ejaculation. A more recent study of men found an increase in plasma oxytocin immediately after orgasm, but only in a portion of their sample that did not reach statistical significance. The authors noted these changes "may simply reflect contractile properties on reproductive tissue".
- Due to its similarity to vasopressin, it can reduce the excretion of urine slightly, and so it can be classified as an antidiuretic. In several species, oxytocin can stimulate sodium excretion from the kidneys (natriuresis), and, in humans, high doses can result in low sodium levels (hyponatremia).
- Cardiac effects: oxytocin and oxytocin receptors are also found in the heart in some rodents, and the hormone may play a role in the embryonal development of the heart by promoting cardiomyocyte differentiation. However, the absence of either oxytocin or its receptor in knockout mice has not been reported to produce cardiac insufficiencies.
- Modulation of hypothalamic-pituitary-adrenal axis activity: oxytocin, under certain circumstances, indirectly inhibits release of adrenocorticotropic hormone and cortisol and, in those situations, may be considered an antagonist of vasopressin.
- Preparing fetal neurons for delivery (in rats): crossing the placenta, maternal oxytocin reaches the fetal brain and induces a switch in the action of neurotransmitter GABA from excitatory to inhibitory on fetal cortical neurons. This silences the fetal brain for the period of delivery and reduces its vulnerability to hypoxic damage.
- Feeding: a 2012 paper suggested that oxytocin neurons in the para-ventricular hypothalamus in the brain may play a key role in suppressing appetite under normal conditions and that other hypothalamic neurons may trigger eating via inhibition of these oxytocin neurons. This population of oxytocin neurons is absent in Prader-Willi syndrome, a genetic disorder that leads to uncontrollable feeding and obesity, and may play a key role in its pathophysiology. Research on the oxytocin-related neuropeptide asterotocin in starfish also showed that in echinoderms, the chemical induces muscle relaxation, and in starfish specifically caused the organisms to evert their stomach and react as though feeding on prey, even when none were present.
Psychological
- Autism: Oxytocin has been implicated in the etiology of autism, with one report suggesting autism is correlated to a mutation on the oxytocin receptor gene (OXTR). Studies involving Caucasian, Finnish and Chinese Han families provide support for the relationship of OXTR with autism. Autism may also be associated with an aberrant methylation of OXTR.
Bonding
In the prairie vole, oxytocin released into the brain of the female during sexual activity is important for forming a pair bond with her sexual partner. Vasopressin appears to have a similar effect in males. Oxytocin has a role in social behaviors in many species, so it likely also does in humans. In a 2003 study, both humans and dog oxytocin levels in the blood rose after five to 24 minutes of a petting session. This possibly plays a role in the emotional bonding between humans and dogs.
- Maternal behavior: Female rats given oxytocin antagonists after giving birth do not exhibit typical maternal behavior. By contrast, virgin female sheep show maternal behavior toward foreign lambs upon cerebrospinal fluid infusion of oxytocin, which they would not do otherwise. Oxytocin is involved in the initiation of human maternal behavior, not its maintenance; for example, it is higher in mothers after they interact with unfamiliar children rather than their own.
- Human ingroup bonding: Oxytocin can increase positive attitudes, such as bonding, toward individuals with similar characteristics, who then become classified as "in-group" members, whereas individuals who are dissimilar become classified as "out-group" members. Race can be used as an example of in-group and out-group tendencies because society often categorizes individuals into groups based on race (Caucasian, African American, Latino, etc.). One study that examined race and empathy found that participants receiving nasally administered oxytocin had stronger reactions to pictures of in-group members making pained faces than to pictures of out-group members with the same expression. Moreover, individuals of one race may be more inclined to help individuals of the same race than individuals of another race when they are experiencing pain. Oxytocin has also been implicated in lying when lying would prove beneficial to other in-group members. In a study where such a relationship was examined, it was found that when individuals were administered oxytocin, rates of dishonesty in the participants' responses increased for their in-group members when a beneficial outcome for their group was expected. Both of these examples show the tendency of individuals to act in ways that benefit those considered to be members of their social group, or in-group.
Oxytocin is not only correlated with the preferences of individuals to associate with members of their own group, but it is also evident during conflicts between members of different groups. During conflict, individuals receiving nasally administered oxytocin demonstrate more frequent defense-motivated responses toward in-group members than out-group members. Further, oxytocin was correlated with participant desire to protect vulnerable in-group members, despite that individual's attachment to the conflict. Similarly, it has been demonstrated that when oxytocin is administered, individuals alter their subjective preferences in order to align with in-group ideals over out-group ideals. These studies demonstrate that oxytocin is associated with intergroup dynamics. Further, oxytocin influences the responses of individuals in a particular group to those of another group. The in-group bias is evident in smaller groups; however, it can also be extended to groups as large as one's entire country leading toward a tendency of strong national zeal. A study done in the Netherlands showed that oxytocin increased the in-group favoritism of their nation while decreasing acceptance of members of other ethnicities and foreigners. People also show more affection for their country's flag while remaining indifferent to other cultural objects when exposed to oxytocin. It has thus been hypothesized that this hormone may be a factor in xenophobic tendencies secondary to this effect. Thus, oxytocin appears to affect individuals at an international level where the in-group becomes a specific "home" country and the out-group grows to include all other countries.
Drugs
- Drug interaction: According to several studies in animals, oxytocin inhibits the development of tolerance to various addictive drugs (opiates, cocaine, alcohol), and reduces withdrawal symptoms. MDMA (ecstasy) may increase feelings of love, empathy, and connection to others by stimulating oxytocin activity primarily via activation of serotonin 5-HT1A receptors, if initial studies in animals apply to humans. The anxiolytic drug buspirone may produce some of its effects via 5-HT1A receptor-induced oxytocin stimulation as well.
- Addiction vulnerability: Concentrations of endogenous oxytocin can impact the effects of various drugs and one's susceptibility to substance use disorders, with higher concentrations associated with lower susceptibility. The status of the endogenous oxytocin system can enhance or reduce susceptibility to addiction through its bidirectional interaction with numerous systems, including the dopamine system, the hypothalamic–pituitary–adrenal axis and the immune system. Individual differences in the endogenous oxytocin system based on genetic predisposition, gender and environmental influences, may therefore affect addiction vulnerability. Oxytocin may be related to the place conditioning behaviors observed in habitual drug abusers.
Fear and anxiety
Oxytocin is typically remembered for the effect it has on prosocial behaviors, such as its role in facilitating trust and attachment between individuals. However, oxytocin has a more complex role than solely enhancing prosocial behaviors. There is consensus that oxytocin modulates fear and anxiety; that is, it does not directly elicit fear or anxiety. Two dominant theories explain the role of oxytocin in fear and anxiety. One theory states that oxytocin increases approach/avoidance to certain social stimuli and the second theory states that oxytocin increases the salience of certain social stimuli, causing the animal or human to pay closer attention to socially relevant stimuli.
Nasally administered oxytocin has been reported to reduce fear, possibly by inhibiting the amygdala (which is thought to be responsible for fear responses). Indeed, studies in rodents have shown oxytocin can efficiently inhibit fear responses by activating an inhibitory circuit within the amygdala. Some researchers have argued oxytocin has a general enhancing effect on all social emotions, since intranasal administration of oxytocin also increases envy and Schadenfreude. Individuals who receive an intranasal dose of oxytocin identify facial expressions of disgust more quickly than individuals who do not receive oxytocin. Facial expressions of disgust are evolutionarily linked to the idea of contagion. Thus, oxytocin increases the salience of cues that imply contamination, which leads to a faster response because these cues are especially relevant for survival. In another study, after administration of oxytocin, individuals displayed an enhanced ability to recognize expressions of fear compared to the individuals who received the placebo. Oxytocin modulates fear responses by enhancing the maintenance of social memories. Rats that are genetically modified to have a surplus of oxytocin receptors display a greater fear response to a previously conditioned stressor. Oxytocin enhances the aversive social memory, leading the rat to display a greater fear response when the aversive stimulus is encountered again.
Mood and depression
Oxytocin produces antidepressant-like effects in animal models of depression, and a deficit of it may be involved in the pathophysiology of depression in humans. The antidepressant-like effects of oxytocin are not blocked by a selective antagonist of the oxytocin receptor, suggesting that these effects are not mediated by the oxytocin receptor. In accordance, unlike oxytocin, the selective non-peptide oxytocin receptor agonist WAY-267,464 does not produce antidepressant-like effects, at least in the tail suspension test. In contrast to WAY-267,464, carbetocin, a close analogue of oxytocin and peptide oxytocin receptor agonist, notably does produce antidepressant-like effects in animals. As such, the antidepressant-like effects of oxytocin may be mediated by modulation of a different target, perhaps the vasopressin V1A receptor where oxytocin is known to weakly bind as an agonist.
Sildenafil enhances electrically evoked oxytocin release from the pituitary gland. In accordance, it may have promise as an antidepressant.
Sex differences
It has been shown that oxytocin differentially affects males and females. Females who are administered oxytocin are overall faster in responding to socially relevant stimuli than males who received oxytocin. Additionally, after the administration of oxytocin, females show increased amygdala activity in response to threatening scenes; however, males do not show increased amygdala activation. This phenomenon can be explained by looking at the role of gonadal hormones, specifically estrogen, which modulate the enhanced threat processing seen in females. Estrogen has been shown to stimulate the release of oxytocin from the hypothalamus and promote receptor binding in the amygdala.
It has also been shown that testosterone directly suppresses oxytocin in mice. This has been hypothesized to have evolutionary significance. With oxytocin suppressed, activities such as hunting and attacking invaders would be less mentally difficult as oxytocin is strongly associated with empathy.
Social
- Affecting generosity by increasing empathy during perspective taking: In a neuroeconomics experiment, intranasal oxytocin increased generosity in the Ultimatum Game by 80%, but had no effect in the Dictator Game that measures altruism. Perspective-taking is not required in the Dictator Game, but the researchers in this experiment explicitly induced perspective-taking in the Ultimatum Game by not identifying to participants into which role they would be placed. Serious methodological questions have arisen, however, with regard to the role of oxytocin in trust and generosity. Empathy in healthy males has been shown to be increased after intranasal oxytocin. This is most likely due to the effect of oxytocin in enhancing eye gaze. There is some discussion about which aspect of empathy oxytocin might alter – for example, cognitive vs. emotional empathy. While studying wild chimpanzees, it was noted that after a chimpanzee shared food with a non-kin related chimpanzee, the subjects' levels of oxytocin increased, as measured through their urine. In comparison to other cooperative activities between chimpanzees that were monitored including grooming, food sharing generated higher levels of oxytocin. This comparatively higher level of oxytocin after food sharing parallels the increased level of oxytocin in nursing mothers, sharing nutrients with their kin.
- Trust is increased by oxytocin. Disclosure of emotional events is a sign of trust in humans. When recounting a negative event, humans who receive intranasal oxytocin share more emotional details and stories with more emotional significance. Humans also find faces more trustworthy after receiving intranasal oxytocin. In a study, participants who received intranasal oxytocin viewed photographs of human faces with neutral expressions and found them to be more trustworthy than those who did not receive oxytocin. This may be because oxytocin reduces the fear of social betrayal in humans. Even after experiencing social alienation by being excluded from a conversation, humans who received oxytocin scored higher in trust on the Revised NEO Personality Inventory. Moreover, in a risky investment game, experimental subjects given nasally administered oxytocin displayed "the highest level of trust" twice as often as the control group. Subjects who were told they were interacting with a computer showed no such reaction, leading to the conclusion that oxytocin was not merely affecting risk aversion. When there is a reason to be distrustful, such as experiencing betrayal, differing reactions are associated with oxytocin receptor gene (OXTR) differences. Those with the CT haplotype experience a stronger reaction, in the form of anger, to betrayal.
- Romantic attachment: In some studies, high levels of plasma oxytocin have been correlated with romantic attachment. For example, if a couple is separated for a long period of time, anxiety can increase due to the lack of physical affection. Oxytocin may aid romantically attached couples by decreasing their feelings of anxiety when they are separated.
- Group-serving dishonesty/deception: In a carefully controlled study exploring the biological roots of immoral behavior, oxytocin was shown to promote dishonesty when the outcome favored the group to which an individual belonged instead of just the individual.
- Oxytocin affects social distance between adult males and females, and may be responsible at least in part for romantic attraction and subsequent monogamous pair bonding. An oxytocin nasal spray caused men in a monogamous relationship, but not single men, to increase the distance between themselves and an attractive woman during a first encounter by 10 to 15 centimeters. The researchers suggested that oxytocin may help promote fidelity within monogamous relationships. For this reason, it is sometimes referred to as the "bonding hormone". There is some evidence that oxytocin promotes ethnocentric behavior, incorporating the trust and empathy of in-groups with their suspicion and rejection of outsiders. Furthermore, genetic differences in the oxytocin receptor gene (OXTR) have been associated with maladaptive social traits such as aggressive behavior.
- Social behavior and wound healing: Oxytocin is also thought to modulate inflammation by decreasing certain cytokines. Thus, the increased release in oxytocin following positive social interactions has the potential to improve wound healing. A study by Marazziti and colleagues used heterosexual couples to investigate this possibility. They found increases in plasma oxytocin following a social interaction were correlated with faster wound healing. They hypothesized this was due to oxytocin reducing inflammation, thus allowing the wound to heal more quickly. This study provides preliminary evidence that positive social interactions may directly influence aspects of health. According to a study published in 2014, silencing of oxytocin receptor interneurons in the medial prefrontal cortex (mPFC) of female mice resulted in loss of social interest in male mice during the sexually receptive phase of the estrous cycle. Oxytocin evokes feelings of contentment, reductions in anxiety, and feelings of calmness and security when in the company of the mate. This suggests oxytocin may be important for the inhibition of the brain regions associated with behavioral control, fear, and anxiety, thus allowing orgasm to occur. Research has also demonstrated that oxytocin can decrease anxiety and protect against stress, particularly in combination with social support. It is found, that endocannabinoid signaling mediates oxytocin-driven social reward.
Chemistry
Oxytocin is a peptide of nine amino acids (a nonapeptide) in the sequence cysteine-tyrosine-isoleucine-glutamine-asparagine-cysteine-proline-leucine-glycine-amide (Cys – Tyr – Ile – Gln – Asn – Cys – Pro – Leu – Gly – NH2, or CYIQNCPLG-NH2); its C-terminus has been converted to a primary amide and a disulfide bridge joins the cysteine moieties. Oxytocin has a molecular mass of 1007 Da, and one international unit (IU) of oxytocin is the equivalent of 1.68 μg of pure peptide.
While the structure of oxytocin is highly conserved in placental mammals, a novel structure of oxytocin was recently reported in marmosets, tamarins, and other new world primates. Genomic sequencing of the gene for oxytocin revealed a single in-frame mutation (thymine for cytosine) which results in a single amino acid substitution at the 8-position (proline for leucine). Since this original Lee et al. paper, two other laboratories have confirmed Pro8-OT and documented additional oxytocin structural variants in this primate taxon. Vargas-Pinilla et al. sequenced the coding regions of the OXT gene in other genera in new world primates and identified the following variants in addition to Leu8- and Pro8-OT: Ala8-OT, Thr8-OT, and Val3/Pro8-OT. Ren et al. identified a variant further, Phe2-OT in howler monkeys.
The biologically active form of oxytocin, commonly measured by RIA and/or HPLC techniques, is the oxidized octapeptide oxytocin disulfide, but oxytocin also exists as a reduced straight-chain (non-cyclic) dithiol nonapeptide called oxytoceine. It has been theorized that oxytoceine may act as a free radical scavenger, as donating an electron to a free radical allows oxytoceine to be re-oxidized to oxytocin via the dehydroascorbate / ascorbate redox couple.
Recent advances in analytical instrumental techniques highlighted the importance of liquid chromatography (LC) coupled with mass spectrometry (MS) for measuring oxytocin levels in various samples derived from biological sources. Most of these studies optimized the oxytocin quantification in electrospray ionization (ESI) positive mode, using [M+H]+ as the parent ion at mass-to-charge ratio (m/z) 1007.4 and the fragment ions as diagnostic peaks at m/z 991.0, m/z 723.2 and m/z 504.2. These important ion selections paved the way for the development of current methods of oxytocin quantification using MS instrumentation.
The structure of oxytocin is very similar to that of vasopressin. Both are nonapeptides with a single disulfide bridge, differing only by two substitutions in the amino acid sequence (differences from oxytocin bolded for clarity): Cys – Tyr – Phe – Gln – Asn – Cys – Pro – Arg – Gly – NH2. Oxytocin and vasopressin were isolated and their total synthesis reported in 1954, work for which Vincent du Vigneaud was awarded the 1955 Nobel Prize in Chemistry with the citation: "for his work on biochemically important sulphur compounds, especially for the first synthesis of a polypeptide hormone."
Oxytocin and vasopressin are the only known hormones released by the human posterior pituitary gland to act at a distance. However, oxytocin neurons make other peptides, including corticotropin-releasing hormone and dynorphin, for example, that act locally. The magnocellular neurosecretory cells that make oxytocin are adjacent to magnocellular neurosecretory cells that make vasopressin. These are large neuroendocrine neurons which are excitable and can generate action potentials.
History
The uterine-contracting properties of the principle that would later be named oxytocin were discovered by British pharmacologist Sir Henry Hallett Dale in 1906, and its milk ejection property was described by Ott and Scott in 1910 and by Schafer and Mackenzie in 1911. In the 1920s, oxytocin and vasopressin were isolated from pituitary tissue and given their current names. The word oxytocin was coined from the term oxytocic, Greek ὀξύς, oxys, meaning "sharp" or "swift", and τόκος, toκos, meaning "childbirth".
Oxytocin became the first polypeptide hormone to be sequenced or synthesized. Du Vigneaud was awarded the Nobel Prize in 1955 for his work.
Further work on different synthetic routes for oxytocin, as well as the preparation of analogues of the hormone (e.g. 4-deamido-oxytocin) was performed in the following decade by Iphigenia Photaki.