Proteins are essential nutrients for the human body. They are one of the building blocks of body tissue and can also serve as a fuel source. As a fuel, proteins provide as much energy density as carbohydrates: 4 kcal (17 kJ) per gram; in contrast, lipids provide 9 kcal (37 kJ) per gram. The most important aspect and defining characteristic of protein from a nutritional standpoint is its amino acid composition.
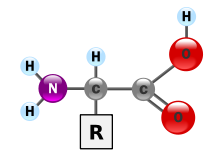
Proteins are polymer chains made of amino acids linked together by peptide bonds. During human digestion, proteins are broken down in the stomach to smaller polypeptide chains via hydrochloric acid and protease actions. This is crucial for the absorption of the essential amino acids that cannot be biosynthesized by the body.
There are nine essential amino acids which humans must obtain from their diet in order to prevent protein–energy malnutrition and resulting death. They are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine. There has been debate as to whether there are 8 or 9 essential amino acids. The consensus seems to lean towards 9 since histidine is not synthesized in adults. There are five amino acids which humans are able to synthesize in the body. These five are alanine, aspartic acid, asparagine, glutamic acid and serine. There are six conditionally essential amino acids whose synthesis can be limited under special pathophysiological conditions, such as prematurity in the infant or individuals in severe catabolic distress. These six are arginine, cysteine, glycine, glutamine, proline and tyrosine. Dietary sources of protein include meats, dairy products, fish, eggs, grains, legumes, nuts and edible insects.
Protein functions in human body
Protein is a nutrient needed by the human body for growth and maintenance. Aside from water, proteins are the most abundant kind of molecules in the body. Protein can be found in all cells of the body and is the major structural component of all cells in the body, especially muscle. This also includes body organs, hair and skin. Proteins are also used in membranes, such as glycoproteins. When broken down into amino acids, they are used as precursors to nucleic acid, co-enzymes, hormones, immune response, cellular repair, and other molecules essential for life. Additionally, protein is needed to form blood cells.
Sources
| Categories | Contribution of farmed animal product [%] |
|---|---|
| Calories | 18
|
| Proteins | 37
|
| Land use | 83
|
| Greenhouse gases | 58
|
| Water pollution | 57
|
| Air pollution | 56
|
| Freshwater withdrawals | 33
|
Protein occurs in a wide range of food. On a worldwide basis, plant protein foods contribute over 60% of the per capita supply of protein. In North America, animal-derived foods contribute about 70% of protein sources. Insects are a source of protein in many parts of the world. In parts of Africa, up to 50% of dietary protein derives from insects. It is estimated that more than 2 billion people eat insects daily.
Meat, dairy, eggs, soy, fish, whole grains, and cereals are sources of protein. Examples of food staples and cereal sources of protein, each with a concentration greater than 7%, are (in no particular order) buckwheat, oats, rye, millet, maize (corn), rice, wheat, sorghum, amaranth, and quinoa. Some research highlights game meat as a protein source.
Vegan sources of proteins include legumes, nuts, seeds and fruits. Vegan foods with protein concentrations greater than 7% include soybeans, lentils, kidney beans, white beans, mung beans, chickpeas, cowpeas, lima beans, pigeon peas, lupines, wing beans, almonds, Brazil nuts, cashews, pecans, walnuts, cotton seeds, pumpkin seeds, hemp seeds, sesame seeds, and sunflower seeds.
Photovoltaic-driven microbial protein production uses electricity from solar panels and carbon dioxide from the air to create fuel for microbes, which are grown in bioreactor vats and then processed into dry protein powders. The process makes highly efficient use of land, water and fertiliser.
People eating a balanced diet do not need protein supplements.
The table below presents food groups as protein sources.
| Food source | Lysine | Threonine | Tryptophan | Sulfur-containing amino acids |
|---|---|---|---|---|
| Legumes | 64 | 38 | 12 | 25 |
| Cereals and whole grains | 31 | 32 | 12 | 37 |
| Nuts and seeds | 45 | 36 | 17 | 46 |
| Fruits | 45 | 29 | 11 | 27 |
| Animal | 85 | 44 | 12 | 38 |
Colour key:
- Protein source with highest density of respective amino acid.
- Protein source with lowest density of respective amino acid.
Protein powders – such as casein, whey, egg, rice, soy and cricket flour– are processed and manufactured sources of protein.
Testing in foods
The classic assays for protein concentration in food are the Kjeldahl method and the Dumas method. These tests determine the total nitrogen in a sample. The only major component of most food which contains nitrogen is protein (fat, carbohydrate and dietary fiber do not contain nitrogen). If the amount of nitrogen is multiplied by a factor depending on the kinds of protein expected in the food the total protein can be determined. This value is known as the "crude protein" content. On food labels the protein is given by the nitrogen multiplied by 6.25, because the average nitrogen content of proteins is about 16%. The Kjeldahl test is typically used because it is the method the AOAC International has adopted and is therefore used by many food standards agencies around the world, though the Dumas method is also approved by some standards organizations.
Accidental contamination and intentional adulteration of protein meals with non-protein nitrogen sources that inflate crude protein content measurements have been known to occur in the food industry for decades. To ensure food quality, purchasers of protein meals routinely conduct quality control tests designed to detect the most common non-protein nitrogen contaminants, such as urea and ammonium nitrate.
In at least one segment of the food industry, the dairy industry, some countries (at least the U.S., Australia, France and Hungary) have adopted "true protein" measurement, as opposed to crude protein measurement, as the standard for payment and testing: "True protein is a measure of only the proteins in milk, whereas crude protein is a measure of all sources of nitrogen and includes nonprotein nitrogen, such as urea, which has no food value to humans. ... Current milk-testing equipment measures peptide bonds, a direct measure of true protein." Measuring peptide bonds in grains has also been put into practice in several countries including Canada, the UK, Australia, Russia and Argentina where near-infrared reflectance (NIR) technology, a type of infrared spectroscopy is used. The Food and Agriculture Organization of the United Nations (FAO) recommends that only amino acid analysis be used to determine protein in, inter alia, foods used as the sole source of nourishment, such as infant formula, but also provides: "When data on amino acids analyses are not available, determination of protein based on total N content by Kjeldahl (AOAC, 2000) or similar method ... is considered acceptable."
The testing method for protein in beef cattle feed has grown into a science over the post-war years. The standard text in the United States, Nutrient Requirements of Beef Cattle, has been through eight editions over at least seventy years. The 1996 sixth edition substituted for the fifth edition's crude protein the concept of "metabolizeable protein", which was defined around the year 2000 as "the true protein absorbed by the intestine, supplied by microbial protein and undegraded intake protein".
The limitations of the Kjeldahl method were at the heart of the Chinese protein export contamination in 2007 and the 2008 China milk scandal in which the industrial chemical melamine was added to the milk or glutens to increase the measured "protein".
Protein quality
The most important aspect and defining characteristic of protein from a nutritional standpoint is its amino acid composition. There are multiple systems which rate proteins by their usefulness to an organism based on their relative percentage of amino acids and, in some systems, the digestibility of the protein source. They include biological value, net protein utilization, and PDCAAS (Protein Digestibility Corrected Amino Acids Score) which was developed by the FDA as a modification of the Protein efficiency ratio (PER) method. The PDCAAS rating was adopted by the US Food and Drug Administration (FDA) and the Food and Agricultural Organization of the United Nations/World Health Organization (FAO/WHO) in 1993 as "the preferred 'best'" method to determine protein quality. These organizations have suggested that other methods for evaluating the quality of protein are inferior. In 2013 FAO proposed changing to Digestible Indispensable Amino Acid Score.
Digestion
Most proteins are decomposed to single amino acids by digestion in the gastro-intestinal tract.
Digestion typically begins in the stomach when pepsinogen is converted to pepsin by the action of hydrochloric acid, and continued by trypsin and chymotrypsin in the small intestine. Before the absorption in the small intestine, most proteins are already reduced to single amino acid or peptides of several amino acids. Most peptides longer than four amino acids are not absorbed. Absorption into the intestinal absorptive cells is not the end. There, most of the peptides are broken into single amino acids.
Absorption of the amino acids and their derivatives into which dietary protein is degraded is done by the gastrointestinal tract. The absorption rates of individual amino acids are highly dependent on the protein source; for example, the digestibilities of many amino acids in humans, the difference between soy and milk proteins and between individual milk proteins, beta-lactoglobulin and casein. For milk proteins, about 50% of the ingested protein is absorbed between the stomach and the jejunum and 90% is absorbed by the time the digested food reaches the ileum. Biological value (BV) is a measure of the proportion of absorbed protein from a food which becomes incorporated into the proteins of the organism's body.
Newborn
Newborns of mammals are exceptional in protein digestion and assimilation in that they can absorb intact proteins at the small intestine. This enables passive immunity, i.e., transfer of immunoglobulins from the mother to the newborn, via milk.
Dietary requirements
Considerable debate has taken place regarding issues surrounding protein intake requirements. The amount of protein required in a person's diet is determined in large part by overall energy intake, the body's need for nitrogen and essential amino acids, body weight and composition, rate of growth in the individual, physical activity level, the individual's energy and carbohydrate intake, and the presence of illness or injury. Physical activity and exertion as well as enhanced muscular mass increase the need for protein. Requirements are also greater during childhood for growth and development, during pregnancy, or when breastfeeding in order to nourish a baby or when the body needs to recover from malnutrition or trauma or after an operation.
Dietary recommendations
According to US & Canadian Dietary Reference Intake guidelines, women aged 19–70 need to consume 46 grams of protein per day while men aged 19–70 need to consume 56 grams of protein per day to minimize risk of deficiency. These Recommended Dietary Allowances (RDAs) were calculated based on 0.8 grams protein per kilogram body weight and average body weights of 57 kg (126 pounds) and 70 kg (154 pounds), respectively. However, this recommendation is based on structural requirements but disregards use of protein for energy metabolism. This requirement is for a normal sedentary person. In the United States, average protein consumption is higher than the RDA. According to results of the National Health and Nutrition Examination Survey (NHANES 2013-2014), average protein consumption for women ages 20 and older was 69.8 grams and for men 98.3 grams/day.
Active people
Several studies have concluded that active people and athletes may require elevated protein intake (compared to 0.8 g/kg) due to increase in muscle mass and sweat losses, as well as need for body repair and energy source. Suggested amounts vary from 1.2 to 1.4 g/kg for those doing endurance exercise to as much as 1.6-1.8 g/kg for strength exercise, while a proposed maximum daily protein intake would be approximately 25% of energy requirements i.e. approximately 2 to 2.5 g/kg. However, many questions still remain to be resolved.
In addition, some have suggested that athletes using restricted-calorie diets for weight loss should further increase their protein consumption, possibly to 1.8–2.0 g/kg, in order to avoid loss of lean muscle mass.
Aerobic exercise protein needs
Endurance athletes differ from strength-building athletes in that endurance athletes do not build as much muscle mass from training as strength-building athletes do. Research suggests that individuals performing endurance activity require more protein intake than sedentary individuals so that muscles broken down during endurance workouts can be repaired. Although the protein requirement for athletes still remains controversial (for instance see Lamont, Nutrition Research Reviews, pages 142 - 149, 2012), research does show that endurance athletes can benefit from increasing protein intake because the type of exercise endurance athletes participate in still alters the protein metabolism pathway. The overall protein requirement increases because of amino acid oxidation in endurance-trained athletes. Endurance athletes who exercise over a long period (2–5 hours per training session) use protein as a source of 5–10% of their total energy expended. Therefore, a slight increase in protein intake may be beneficial to endurance athletes by replacing the protein lost in energy expenditure and protein lost in repairing muscles. One review concluded that endurance athletes may increase daily protein intake to a maximum of 1.2–1.4 g per kg body weight.
Anaerobic exercise protein needs
Research also indicates that individuals performing strength training activity require more protein than sedentary individuals. Strength-training athletes may increase their daily protein intake to a maximum of 1.4–1.8 g per kg body weight to enhance muscle protein synthesis, or to make up for the loss of amino acid oxidation during exercise. Many athletes maintain a high-protein diet as part of their training. In fact, some athletes who specialize in anaerobic sports (e.g., weightlifting) believe a very high level of protein intake is necessary, and so consume high protein meals and also protein supplements.
Special populations
Protein allergies
A food allergy is an abnormal immune response to proteins in food. The signs and symptoms may range from mild to severe. They may include itchiness, swelling of the tongue, vomiting, diarrhea, hives, trouble breathing, or low blood pressure. These symptoms typically occurs within minutes to one hour after exposure. When the symptoms are severe, it is known as anaphylaxis. The following eight foods are responsible for about 90% of allergic reactions: cow's milk, eggs, wheat, shellfish, fish, peanuts, tree nuts and soy.
Chronic kidney disease
While there is no conclusive evidence that a high protein diet can cause chronic kidney disease, there is a consensus that people with this disease should decrease consumption of protein. According to one 2009 review updated in 2018, people with chronic kidney disease who reduce protein consumption have less likelihood of progressing to end stage kidney disease. Moreover, people with this disease while using a low protein diet (0.6 g/kg/d - 0.8 g/kg/d) may develop metabolic compensations that preserve kidney function, although in some people, malnutrition may occur.
Phenylketonuria
Individuals with phenylketonuria (PKU) must keep their intake of phenylalanine - an essential amino acid - extremely low to prevent a mental disability and other metabolic complications. Phenylalanine is a component of the artificial sweetener aspartame, so people with PKU need to avoid low calorie beverages and foods with this ingredient.
Maple syrup urine disease
Maple syrup urine disease is associated with genetic anomalies in the metabolism of branched-chain amino acids (BCAAs). They have high blood levels of BCAAs and must severely restrict their intake of BCAAs in order to prevent mental retardation and death. The amino acids in question are leucine, isoleucine and valine. The condition gets its name from the distinctive sweet odor of affected infants' urine. Children of Amish, Mennonite, and Ashkenazi Jewish descent have a high prevalence of this disease compared to other populations.
Excess consumption
The U.S. and Canadian Dietary Reference Intake review for protein concluded that there was not sufficient evidence to establish a Tolerable upper intake level, i.e., an upper limit for how much protein can be safely consumed.
When amino acids are in excess of needs, the liver takes up the amino acids and deaminates them, a process converting the nitrogen from the amino acids into ammonia, further processed in the liver into urea via the urea cycle. Excretion of urea occurs via the kidneys. Other parts of the amino acid molecules can be converted into glucose and used for fuel. When food protein intake is periodically high or low, the body tries to keep protein levels at an equilibrium by using the "labile protein reserve" to compensate for daily variations in protein intake. However, unlike body fat as a reserve for future caloric needs, there is no protein storage for future needs.
Excessive protein intake may increase calcium excretion in urine, occurring to compensate for the pH imbalance from oxidation of sulfur amino acids. This may lead to a higher risk of kidney stone formation from calcium in the renal circulatory system. One meta-analysis reported no adverse effects of higher protein intakes on bone density. Another meta-analysis reported a small decrease in systolic and diastolic blood pressure with diets higher in protein, with no differences between animal and plant protein.
High protein diets have been shown to lead to an additional 1.21 kg of weight loss over a period of 3 months versus a baseline protein diet in a meta-analysis. Benefits of decreased body mass index as well as HDL cholesterol were more strongly observed in studies with only a slight increase in protein intake rather where high protein intake was classified as 45% of total energy intake. Detrimental effects to cardiovascular activity were not observed in short-term diets of 6 months or less. There is little consensus on the potentially detrimental effects to healthy individuals of a long-term high protein diet, leading to caution advisories about using high protein intake as a form of weight loss.
The 2015–2020 Dietary Guidelines for Americans (DGA) recommends that men and teenage boys increase their consumption of fruits, vegetables and other under-consumed foods, and that a means of accomplishing this would be to reduce overall intake of protein foods. The 2015 - 2020 DGA report does not set a recommended limit for the intake of red and processed meat. While the report acknowledges research showing that lower intake of red and processed meat is correlated with reduced risk of cardiovascular diseases in adults, it also notes the value of nutrients provided from these meats. The recommendation is not to limit intake of meats or protein, but rather to monitor and keep within daily limits the sodium (< 2300 mg), saturated fats (less than 10% of total calories per day), and added sugars (less than 10% of total calories per day) that may be increased as a result of consumption of certain meats and proteins. While the 2015 DGA report does advise for a reduced level of consumption of red and processed meats, the 2015-2020 DGA key recommendations recommend that a variety of protein foods be consumed, including both vegetarian and non-vegetarian sources of protein.
Protein deficiency
Protein deficiency and malnutrition (PEM) can lead to variety of ailments including Intellectual disability and kwashiorkor. Symptoms of kwashiorkor include apathy, diarrhea, inactivity, failure to grow, flaky skin, fatty liver, and edema of the belly and legs. This edema is explained by the action of lipoxygenase on arachidonic acid to form leukotrienes and the normal functioning of proteins in fluid balance and lipoprotein transport.
PEM is fairly common worldwide in both children and adults and accounts for 6 million deaths annually. In the industrialized world, PEM is predominantly seen in hospitals, is associated with disease, or is often found in the elderly.