|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
| IUPAC name
Azane
|
|||
| Other names
Hydrogen nitride
Trihydrogen nitride Nitrogen trihydride |
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| 3DMet | B00004 | ||
| 3587154 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.760 | ||
| EC Number | 231-635-3 | ||
| 79 | |||
| KEGG | |||
| MeSH | Ammonia | ||
|
PubChem CID
|
|||
| RTECS number | BO0875000 | ||
| UNII | |||
| UN number | 1005 | ||
| Properties | |||
| NH3 | |||
| Molar mass | 17.031 g/mol | ||
| Appearance | Colourless gas | ||
| Odor | strong pungent odour | ||
| Density | 0.86 kg/m3 (1.013 bar at boiling point) 0.769 kg/m3 (STP)[1] 0.73 kg/m3 (1.013 bar at 15 °C) 681.9 kg/m3 at −33.3 °C (liquid)[2] See also Ammonia (data page) 817 kg/m3 at −80 °C (transparent solid)[3] |
||
| Melting point | −77.73 °C (−107.91 °F; 195.42 K) (Triple point at 6.060 kPa, 195.4 K) | ||
| Boiling point | −33.34 °C (−28.01 °F; 239.81 K) | ||
| 47% w/w (0 °C) 31% w/w (25 °C) 18% w/w (50 °C)[4] |
|||
| Solubility | soluble in chloroform, ether, ethanol, methanol | ||
| Vapor pressure | 857.3 kPa | ||
| Acidity (pKa) | 32.5 (−33 °C),[5] 10.5 (DMSO) | ||
| Basicity (pKb) | 4.75 | ||
| -18.0·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.3327 | ||
| Viscosity | 0.276 cP (−40 °C) | ||
| Structure | |||
| C3v | |||
| Trigonal pyramid | |||
| 1.42 D | |||
| Thermochemistry | |||
|
Std molar
entropy (S |
193 J·mol−1·K−1[6] | ||
|
Std enthalpy of
formation (ΔfH |
−46 kJ·mol−1[6] | ||
| Hazards | |||
| Safety data sheet | See: data page ICSC 0414 (anhydrous) |
||
| GHS pictograms |     [7] [7] |
||
| H221, H280, H314, H331, H400[7] | |||
| P210, P261, P273, P280, P305+351+338, P310[7] | |||
| NFPA 704 | |||
| Flash point | flammable gas | ||
| 651 °C (1,204 °F; 924 K) | |||
| Explosive limits | 15–28% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
0.015 mL/kg (human, oral) | ||
|
LC50 (median concentration)
|
40,300 ppm (rat, 10 min) 28595 ppm (rat, 20 min) 20300 ppm (rat, 40 min) 11590 ppm (rat, 1 hr) 7338 ppm (rat, 1 hr) 4837 ppm (mouse, 1 hr) 9859 ppm (rabbit, 1 hr) 9859 ppm (cat, 1 hr) 2000 ppm (rat, 4 hr) 4230 ppm (mouse, 1 hr)[8] |
||
|
LCLo (lowest published)
|
5000 ppm (mammal, 5 min) 5000 ppm (human, 5 min)[8] |
||
| US health exposure limits (NIOSH):[9] | |||
|
PEL (Permissible)
|
50 ppm (25 ppm ACGIH- TLV; 35 ppm STEL) | ||
|
REL (Recommended)
|
TWA 25 ppm (18 mg/m3) ST 35 ppm (27 mg/m3) | ||
|
IDLH (Immediate danger)
|
300 ppm | ||
| Related compounds | |||
|
Other cations
|
Phosphine Arsine Stibine |
||
|
Related nitrogen hydrides
|
Hydrazine Hydrazoic acid |
||
|
Related compounds
|
Ammonium hydroxide | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
Ammonia or azane is a compound of nitrogen and hydrogen with the formula NH3. The simplest pnictogen hydride, ammonia is a colourless gas with a characteristic pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.
Although common in nature and in wide use, ammonia is both caustic and hazardous in its concentrated form. It is classified as an extremely hazardous substance in the United States, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[10]
The global industrial production of ammonia in 2014 was 176 million tonnes,[11] a 16% increase over the 2006 global industrial production of 152 million tonnes.[12] Industrial ammonia is sold either as ammonia liquor (usually 28% ammonia in water) or as pressurized or refrigerated anhydrous liquid ammonia transported in tank cars or cylinders.[13]
NH3 boils at −33.34 °C (−28.012 °F) at a pressure of one atmosphere, so the liquid must be stored under pressure or at low temperature. Household ammonia or ammonium hydroxide is a solution of NH3 in water. The concentration of such solutions is measured in units of the Baumé scale (density), with 26 degrees baumé (about 30% (by weight) ammonia at 15.5 °C or 59.9 °F) being the typical high-concentration commercial product.[14]
Natural occurrence
Ammonia is found in trace quantities in nature, being produced from the nitrogenous animal and vegetable matter. Ammonia and ammonium salts are also found in small quantities in rainwater, whereas ammonium chloride (sal ammoniac), and ammonium sulfate are found in volcanic districts; crystals of ammonium bicarbonate have been found in Patagonian guano.[15] The kidneys secrete ammonia to neutralize excess acid.[16] Ammonium salts are found distributed through fertile soil and in seawater.Ammonia is also found throughout the Solar System on Mars, Jupiter, Saturn, Uranus, Neptune, and Pluto, among other places: on smaller, icy planets such as Pluto, ammonia can act as a geologically important antifreeze, as a mixture of water and ammonia can have a melting point as low as 173 kelvins if the ammonia concentration is high enough and thus allow such planets to retain internal oceans and active geology at a far lower temperature than would be possible with water alone.[17][18] Substances containing ammonia, or those that are similar to it, are called ammoniacal.
Properties
Ammonia is a colourless gas with a characteristic pungent smell. It is lighter than air, its density being 0.589 times that of air. It is easily liquefied due to the strong hydrogen bonding between molecules; the liquid boils at −33.3 °C (−27.94 °F), and freezes at −77.7 °C (−107.86 °F) to white crystals.[15]Ammonia may be conveniently deodorized by reacting it with either sodium bicarbonate or acetic acid. Both of these reactions form an odourless ammonium salt.
- Solid
- The crystal symmetry is cubic, Pearson symbol cP16, space group P213 No.198, lattice constant 0.5125 nm.[19]
- Liquid
- Liquid ammonia possesses strong ionising powers reflecting its high ε of 22. Liquid ammonia has a very high standard enthalpy change of vaporization (23.35 kJ/mol, cf. water 40.65 kJ/mol, methane 8.19 kJ/mol, phosphine 14.6 kJ/mol) and can therefore be used in laboratories in uninsulated vessels without additional refrigeration. See liquid ammonia as a solvent.
- Solvent properties
- Ammonia is miscible with water. In an aqueous solution, it can be expelled by boiling. The aqueous solution of ammonia is basic. The maximum concentration of ammonia in water (a saturated solution) has a density of 0.880 g/cm3 and is often known as '.880 ammonia'. Ammonia does not burn readily or sustain combustion, except under narrow fuel-to-air mixtures of 15–25% air.
- Combustion
- When mixed with oxygen, it burns with a pale yellowish-green flame. At high temperature and in the presence of a suitable catalyst, ammonia is decomposed into its constituent elements. Ignition occurs when chlorine is passed into ammonia, forming nitrogen and hydrogen chloride; if chlorine is present in excess, then the highly explosive nitrogen trichloride (NCl3) is also formed.
Structure
The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory (VSEPR theory) with an experimentally determined bond angle of 106.7°.[20] The central nitrogen atom has five outer electrons with an additional electron from each hydrogen atom. This gives a total of eight electrons, or four electron pairs that are arranged tetrahedrally. Three of these electron pairs are used as bond pairs, which leaves one lone pair of electrons. The lone pair of electrons repel more strongly than bond pairs, therefore the bond angle is not 109.5°, as expected for a regular tetrahedral arrangement, but 106.7°.[20] The nitrogen atom in the molecule has a lone electron pair, which makes ammonia a base, a proton acceptor. This shape gives the molecule a dipole moment and makes it polar. The molecule's polarity and, especially, its ability to form hydrogen bonds, makes ammonia highly miscible with water. Ammonia is moderately basic, a 1.0 M aqueous solution has a pH of 11.6 and if a strong acid is added to such a solution until the solution is neutral (pH = 7), 99.4% of the ammonia molecules are protonated. Temperature and salinity also affect the proportion of NH4+. The latter has the shape of a regular tetrahedron and is isoelectronic with methane.The ammonia molecule readily undergoes nitrogen inversion at room temperature; a useful analogy is an umbrella turning itself inside out in a strong wind. The energy barrier to this inversion is 24.7 kJ/mol, and the resonance frequency is 23.79 GHz, corresponding to microwave radiation of a wavelength of 1.260 cm. The absorption at this frequency was the first microwave spectrum to be observed.[21]
Amphotericity
One of the most characteristic properties of ammonia is its basicity. Ammonia is considered to be a weak base. It combines with acids to form salts; thus with hydrochloric acid it forms ammonium chloride (sal ammoniac); with nitric acid, ammonium nitrate, etc. Perfectly dry ammonia will not combine with perfectly dry hydrogen chloride; moisture is necessary to bring about the reaction.[22][23] As a demonstration experiment, opened bottles of concentrated ammonia and hydrochloric acid produce clouds of ammonium chloride, which seem to appear "out of nothing" as the salt forms where the two diffusing clouds of molecules meet, somewhere between the two bottles.- NH3 + HCl → NH4Cl
Although ammonia is well known as a weak base, it can also act as an extremely weak acid. It is a protic substance and is capable of formation of amides (which contain the NH2− ion). For example, lithium dissolves in liquid ammonia to give a solution of lithium amide:
- 2Li + 2NH3 → 2LiNH2 + H2
Self-dissociation
Like water, ammonia undergoes molecular autoionisation to form its acid and base conjugates:- 2 NH
3 (aq) ⇌ NH+
4 (aq) + NH−
2 (aq)
4) and amide anions (NH−
2) to be present in solution. At standard pressure and temperature, K=[NH+
4][NH−
2] = 10−30
Combustion
The combustion of ammonia to nitrogen and water is exothermic:- 4 NH3 + 3 O2 → 2 N2 + 6 H2O (g) ΔH°r = −1267.20 kJ/mol (or −316.8 kJ/mol if expressed per mol of NH3)
- 4 NH3 + 5 O2 → 4 NO + 6 H2O
- 2 NO + O2 → 2 NO2
Formation of other compounds
In organic chemistry, ammonia can act as a nucleophile in substitution reactions. Amines can be formed by the reaction of ammonia with alkyl halides, although the resulting -NH2 group is also nucleophilic and secondary and tertiary amines are often formed as byproducts. An excess of ammonia helps minimise multiple substitution, and neutralises the hydrogen halide formed. Methylamine is prepared commercially by the reaction of ammonia with chloromethane, and the reaction of ammonia with 2-bromopropanoic acid has been used to prepare racemic alanine in 70% yield. Ethanolamine is prepared by a ring-opening reaction with ethylene oxide: the reaction is sometimes allowed to go further to produce diethanolamine and triethanolamine.Amides can be prepared by the reaction of ammonia with carboxylic acid derivatives. Acyl chlorides are the most reactive, but the ammonia must be present in at least a twofold excess to neutralise the hydrogen chloride formed. Esters and anhydrides also react with ammonia to form amides. Ammonium salts of carboxylic acids can be dehydrated to amides so long as there are no thermally sensitive groups present: temperatures of 150–200 °C are required.
The hydrogen in ammonia is capable of replacement by metals, thus magnesium burns in the gas with the formation of magnesium nitride Mg3N2, and when the gas is passed over heated sodium or potassium, sodamide, NaNH2, and potassamide, KNH2, are formed.[22] Where necessary in substitutive nomenclature, IUPAC recommendations prefer the name "azane" to ammonia: hence chloramine would be named "chloroazane" in substitutive nomenclature, not "chloroammonia".
Pentavalent ammonia is known as λ5-amine, or more commonly, ammonium hydride. This crystalline solid is only stable under high pressure, and decomposes back into trivalent ammonia and hydrogen gas at normal conditions. This substance was once investigated as a possible solid rocket fuel in 1966.[25]
Ammonia as a ligand

Ball-and-stick model of the tetraamminediaquacopper(II) cation, [Cu(NH3)4(H2O)2]2+
Ammonia can act as a ligand in transition metal complexes. It is a pure σ-donor, in the middle of the spectrochemical series, and shows intermediate hard-soft behaviour. For historical reasons, ammonia is named ammine in the nomenclature of coordination compounds. Some notable ammine complexes include tetraamminediaquacopper(II) ([Cu(NH3)4(H2O)2]2+), a dark blue complex formed by adding ammonia to a solution of copper(II) salts. Tetraamminediaquacopper(II) hydroxide is known as Schweizer's reagent, and has the remarkable ability to dissolve cellulose. Diamminesilver(I) ([Ag(NH3)2]+) is the active species in Tollens' reagent. Formation of this complex can also help to distinguish between precipitates of the different silver halides: silver chloride (AgCl) is soluble in dilute (2M) ammonia solution, silver bromide (AgBr) is only soluble in concentrated ammonia solution, whereas silver iodide (AgI) is insoluble in aqueous ammonia.
Ammine complexes of chromium(III) were known in the late 19th century, and formed the basis of Alfred Werner's revolutionary theory on the structure of coordination compounds. Werner noted only two isomers (fac- and mer-) of the complex [CrCl3(NH3)3] could be formed, and concluded the ligands must be arranged around the metal ion at the vertices of an octahedron. This proposal has since been confirmed by X-ray crystallography.
An ammine ligand bound to a metal ion is markedly more acidic than a free ammonia molecule, although deprotonation in aqueous solution is still rare. One example is the Calomel reaction, where the resulting amidomercury(II) compound is highly insoluble.
- Hg2Cl2 + 2 NH3 → Hg + HgCl(NH2) + NH4+ + Cl−
Detection and determination
Ammonia in solution
Ammonia and ammonium salts can be readily detected, in very minute traces, by the addition of Nessler's solution, which gives a distinct yellow colouration in the presence of the least trace of ammonia or ammonium salts. The amount of ammonia in ammonium salts can be estimated quantitatively by distillation of the salts with sodium or potassium hydroxide, the ammonia evolved being absorbed in a known volume of standard sulfuric acid and the excess of acid then determined volumetrically; or the ammonia may be absorbed in hydrochloric acid and the ammonium chloride so formed precipitated as ammonium hexachloroplatinate, (NH4)2PtCl6.[26]Gaseous ammonia
Sulfur sticks are burnt to detect small leaks in industrial ammonia refrigeration systems. Larger quantities can be detected by warming the salts with a caustic alkali or with quicklime, when the characteristic smell of ammonia will be at once apparent.[26] Ammonia is an irritant and irritation increases with concentration; the permissible exposure limit is 25 ppm, and lethal above 500 ppm.[27] Higher concentrations are hardly detected by conventional detectors, the type of detector is chosen according to the sensitivity required (e.g. semiconductor, catalytic, electrochemical). Holographic sensors have been proposed for detecting concentrations up to 12.5% in volume.[28]Ammoniacal nitrogen (NH3-N)
Ammoniacal nitrogen (NH3-N) is a measure commonly used for testing the quantity of ammonium ions, derived naturally from ammonia, and returned to ammonia via organic processes, in water or waste liquids. It is a measure used mainly for quantifying values in waste treatment and water purification systems, as well as a measure of the health of natural and man made water reserves. It is measured in units of mg/L (milligram per litre).History
This high-pressure reactor was built in 1921 by BASF in Ludwigshafen and was re-erected on the premises of the University of Karlsruhe in Germany.
The ancient Greek historian Herodotus mentioned that there were outcrops of salt in an area of Libya that was inhabited by a people called the "Ammonians" (now: the Siwa oasis in northwestern Egypt, where salt lakes still exist).[29][30] The Greek geographer Strabo also mentioned the salt from this region. However, the ancient authors Dioscorides, Apicius, Arrian, Synesius, and Aëtius of Amida described this salt as forming clear crystals that could be used for cooking and that were essentially rock salt.[31] Hammoniacus sal appears in the writings of Pliny,[32] although it is not known whether the term is identical with the more modern sal ammoniac (ammonium chloride).[15][33][34]
The fermentation of urine by bacteria produces a solution of ammonia; hence fermented urine was used in Classical Antiquity to wash cloth and clothing, to remove hair from hides in preparation for tanning, to serve as a mordant in dying cloth, and to remove rust from iron.[35]
In the form of sal ammoniac (نشادر, nushadir) ammonia was important to the Muslim alchemists as early as the 8th century, first mentioned by the Persian-Arab chemist Jābir ibn Hayyān,[36] and to the European alchemists since the 13th century, being mentioned by Albertus Magnus.[15] It was also used by dyers in the Middle Ages in the form of fermented urine to alter the colour of vegetable dyes. In the 15th century, Basilius Valentinus showed that ammonia could be obtained by the action of alkalis on sal ammoniac.[37] At a later period, when sal ammoniac was obtained by distilling the hooves and horns of oxen and neutralizing the resulting carbonate with hydrochloric acid, the name "spirit of hartshorn" was applied to ammonia.[15][38]
Gaseous ammonia was first isolated by Joseph Black in 1756 by reacting sal ammoniac (Ammonium Chloride) with calcined magnesia (Magnesium Oxide).[39][40] It was isolated again by Peter Woulfe in 1767,[41][42] by Carl Wilhelm Scheele in 1770[43] and by Joseph Priestley in 1773 and was termed by him "alkaline air".[15][44] Eleven years later in 1785, Claude Louis Berthollet ascertained its composition.[45][15]
The Haber–Bosch process to produce ammonia from the nitrogen in the air was developed by Fritz Haber and Carl Bosch in 1909 and patented in 1910. It was first used on an industrial scale in Germany during World War I,[46] following the allied blockade that cut off the supply of nitrates from Chile. The ammonia was used to produce explosives to sustain war efforts.[47]
Before the availability of natural gas, hydrogen as a precursor to ammonia production was produced via the electrolysis of water or using the chloralkali process.
With the advent of the steel industry in the 20th century, ammonia became a byproduct of the production of coking coal.
Uses
Fertilizer
Globally, approximately 88% (as of 2014) of ammonia is used as fertilizers either as its salts, solutions or anhydrously.[11] When applied to soil, it helps provide increased yields of crops such as maize and wheat.[48] 30% of agricultural nitrogen applied in the USA is in the form of anhydrous ammonia and worldwide 110 million tonnes are applied each year.[49]Precursor to nitrogenous compounds
Ammonia is directly or indirectly the precursor to most nitrogen-containing compounds. Virtually all synthetic nitrogen compounds are derived from ammonia. An important derivative is nitric acid. This key material is generated via the Ostwald process by oxidation of ammonia with air over a platinum catalyst at 700–850 °C (1,292–1,562 °F), ~9 atm. Nitric oxide is an intermediate in this conversion:[50]- NH3 + 2 O2 → HNO3 + H2O
Ammonia is also used to make the following compounds:
- Hydrazine, in the Olin Raschig process and the peroxide process
- Hydrogen cyanide, in the BMA process and the Andrussow process
- Hydroxylamine and ammonium carbonate, in the Raschig process
- Phenol, in the Raschig–Hooker process
- Urea, in the Bosch–Meiser urea process and in Wöhler synthesis
- Amino acids, using Strecker amino-acid synthesis
- Acrylonitrile, in the Sohio process
Cleaner
Household ammonia is a solution of NH3 in water (i.e., ammonium hydroxide) used as a general purpose cleaner for many surfaces. Because ammonia results in a relatively streak-free shine, one of its most common uses is to clean glass, porcelain and stainless steel. It is also frequently used for cleaning ovens and soaking items to loosen baked-on grime. Household ammonia ranges in concentration by weight from 5 to 10% ammonia.[51] To verify your ammonia concentration refer to the manufacturers material safety data sheet they are required to share when asked.[52]Fermentation
Solutions of ammonia ranging from 16% to 25% are used in the fermentation industry as a source of nitrogen for microorganisms and to adjust pH during fermentation.Antimicrobial agent for food products
As early as in 1895, it was known that ammonia was "strongly antiseptic ... it requires 1.4 grams per litre to preserve beef tea."[53] In one study, anhydrous ammonia destroyed 99.999% of zoonotic bacteria in 3 types of animal feed, but not silage.[54][non-primary source needed] Anhydrous ammonia is currently used commercially to reduce or eliminate microbial contamination of beef.[55][56] Lean finely textured beef in the beef industry is made from fatty beef trimmings (c. 50–70% fat) by removing the fat using heat and centrifugation, then treating it with ammonia to kill E. coli. The process was deemed effective and safe by the US Department of Agriculture based on a study that found that the treatment reduces E. coli to undetectable levels.[57] There have been safety concerns about the process as well as consumer complaints about the taste and smell of beef treated at optimal levels of ammonia.[58] The level of ammonia in any final product has not come close to toxic levels to humans.Minor and emerging uses
Refrigeration – R717
Because of ammonia's vaporization properties, it is a useful refrigerant.[46] It was commonly used before the popularisation of chlorofluorocarbons (Freons). Anhydrous ammonia is widely used in industrial refrigeration applications and hockey rinks because of its high energy efficiency and low cost. It suffers from the disadvantage of toxicity, which restricts its domestic and small-scale use. Along with its use in modern vapor-compression refrigeration it is used in a mixture along with hydrogen and water in absorption refrigerators. The Kalina cycle, which is of growing importance to geothermal power plants, depends on the wide boiling range of the ammonia–water mixture. Ammonia coolant is also used in the S1 radiator aboard the International Space Station in two loops which are used to regulate the internal temperature and enable temperature dependent experiments.[59][60]For remediation of gaseous emissions
Ammonia is used to scrub SO2 from the burning of fossil fuels, and the resulting product is converted to ammonium sulfate for use as fertilizer. Ammonia neutralizes the nitrogen oxides (NOx) pollutants emitted by diesel engines. This technology, called SCR (selective catalytic reduction), relies on a vanadia-based catalyst.[61]Ammonia may be used to mitigate gaseous spills of phosgene.[62]
As a fuel
Ammoniacal Gas Engine Streetcar in New Orleans drawn by Alfred Waud in 1871.
The raw energy density of liquid ammonia is 11.5 MJ/L,[63] which is about a third that of diesel. Although it can be used as a fuel, for a number of reasons this has never been common or widespread. In addition to direct utilization of ammonia as a fuel in combustion engines there is also the opportunity to convert ammonia back to hydrogen where it can be used to power hydrogen fuel cells or it can be directly used within high temperature fuel cells[64].
Ammonia engines or ammonia motors, using ammonia as a working fluid, have been proposed and occasionally used.[65] The principle is similar to that used in a fireless locomotive, but with ammonia as the working fluid, instead of steam or compressed air. Ammonia engines were used experimentally in the 19th century by Goldsworthy Gurney in the UK and the St. Charles Avenue Streetcar line in New Orleans in the 1870s and 1880s,[66] and during World War II ammonia was used to power buses in Belgium.[67]
Ammonia is sometimes proposed as a practical alternative to fossil fuel for internal combustion engines.[67] Its high octane rating of 120[68] and low flame temperature allows the use of high compression ratios without a penalty of high NOx production. Since ammonia contains no carbon, its combustion cannot produce carbon monoxide, hydrocarbons or soot.
However ammonia cannot be easily used in existing Otto cycle engines because of its very narrow flammability range and there are also other barriers to widespread automobile usage. In terms of raw ammonia supplies, plants would have to be built to increase production levels, requiring significant capital and energy sources. Although it is the second most produced chemical, the scale of ammonia production is a small fraction of world petroleum usage. It could be manufactured from renewable energy sources, as well as coal or nuclear power. The 60 MW Rjukan dam in Telemark, Norway produced ammonia for many years from 1913 producing fertilizer for much of Europe.
Despite this, several tests have been done. In 1981, a Canadian company converted a 1981 Chevrolet Impala to operate using ammonia as fuel.[69][70] In 2007, a University of Michigan pickup powered by ammonia drove from Detroit to San Francisco as part of a demonstration, requiring only one fill-up in Wyoming.[71]
Compared to hydrogen as a fuel, ammonia is much more energy efficient, and it would be a much lower cost to produce, store, and deliver hydrogen as ammonia than as compressed and/or cryogenic hydrogen.[63] The conversion of ammonia to hydrogen via the sodium-amide process,[72] either as a catalyst for combustion or as fuel for a proton exchange membrane fuel cell,[63] is another possibility. Conversion to hydrogen would allow the storage of hydrogen at nearly 18 wt% compared to ~5% for gaseous hydrogen under pressure.
Rocket engines have also been fueled by ammonia. The Reaction Motors XLR99 rocket engine that powered the X-15 hypersonic research aircraft used liquid ammonia. Although not as powerful as other fuels, it left no soot in the reusable rocket engine and its density approximately matches the density of the oxidizer, liquid oxygen, which simplified the aircraft's design.
As a stimulant
Anti-meth sign on tank of anhydrous ammonia, Otley, Iowa.
Anhydrous ammonia is a common farm fertilizer that is also a critical
ingredient in making methamphetamine. In 2005, Iowa used grant money to
give out thousands of locks to prevent criminals from getting into the
tanks.[73]
Ammonia, as the vapor released by smelling salts, has found significant use as a respiratory stimulant. Ammonia is commonly used in the illegal manufacture of methamphetamine through a Birch reduction.[74] The Birch method of making methamphetamine is dangerous because the alkali metal and liquid ammonia are both extremely reactive, and the temperature of liquid ammonia makes it susceptible to explosive boiling when reactants are added.[75]
Textile
Liquid ammonia is used for treatment of cotton materials, giving properties like mercerisation, using alkalis. In particular, it is used for prewashing of wool.[76]Lifting gas
At standard temperature and pressure, ammonia is less dense than atmosphere, and has approximately 60% of the lifting power of hydrogen or helium. Ammonia has sometimes been used to fill weather balloons as a lifting gas. Because of its relatively high boiling point (compared to helium and hydrogen), ammonia could potentially be refrigerated and liquefied aboard an airship to reduce lift and add ballast (and returned to a gas to add lift and reduce ballast).Woodworking
Ammonia has been used to darken quartersawn white oak in Arts & Crafts and Mission-style furniture. Ammonia fumes react with the natural tannins in the wood and cause it to change colours.[77]Safety precautions
The world's longest ammonia pipeline, running from the TogliattiAzot plant in Russia to Odessa in Ukraine.
The U. S. Occupational Safety and Health Administration (OSHA) has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume.[78] NIOSH recently reduced the IDLH from 500 to 300 based on recent more conservative interpretations of original research in 1943. IDLH (Immediately Dangerous to Life and Health) is the level to which a healthy worker can be exposed for 30 minutes without suffering irreversible health effects. Other organizations have varying exposure levels. U.S. Navy Standards [U.S. Bureau of Ships 1962] maximum allowable concentrations (MACs):continuous exposure (60 days): 25 ppm / 1 hour: 400 ppm[79] Ammonia vapour has a sharp, irritating, pungent odour that acts as a warning of potentially dangerous exposure. The average odour threshold is 5 ppm, well below any danger or damage. Exposure to very high concentrations of gaseous ammonia can result in lung damage and death.[78] Although ammonia is regulated in the United States as a non-flammable gas, it still meets the definition of a material that is toxic by inhalation and requires a hazardous safety permit when transported in quantities greater than 13,248 L (3,500 gallons).[80] Household products containing ammonia (i.e., Windex) should never be used in conjunction with products containing bleach, as the resulting chemical reaction produces highly toxic fumes.[81]
Liquid ammonia is dangerous because it is hygroscopic and because it can freeze flesh. See Gas carrier#Health effects of specific cargoes carried on gas carriers for more information.
Toxicity
The toxicity of ammonia solutions does not usually cause problems for humans and other mammals, as a specific mechanism exists to prevent its build-up in the bloodstream. Ammonia is converted to carbamoyl phosphate by the enzyme carbamoyl phosphate synthetase, and then enters the urea cycle to be either incorporated into amino acids or excreted in the urine. [82] Fish and amphibians lack this mechanism, as they can usually eliminate ammonia from their bodies by direct excretion. Ammonia even at dilute concentrations is highly toxic to aquatic animals, and for this reason it is classified as dangerous for the environment.Ammonia is a constituent of tobacco smoke.[83]
Coking wastewater
Ammonia is present in coking wastewater streams, as a liquid by-product of the production of coke from coal.[84] In some cases, the ammonia is discharged to the marine environment where it acts as a pollutant. The Whyalla steelworks in South Australia is one example of a coke-producing facility which discharges ammonia into marine waters.[85]Aquaculture
Ammonia toxicity is believed to be a cause of otherwise unexplained losses in fish hatcheries. Excess ammonia may accumulate and cause alteration of metabolism or increases in the body pH of the exposed organism. Tolerance varies among fish species.[86] At lower concentrations, around 0.05 mg/L, un-ionised ammonia is harmful to fish species and can result in poor growth and feed conversion rates, reduced fecundity and fertility and increase stress and susceptibility to bacterial infections and diseases.[87] Exposed to excess ammonia, fish may suffer loss of equilibrium, hyper-excitability, increased respiratory activity and oxygen uptake and increased heart rate.[86] At concentrations exceeding 2.0 mg/L, ammonia causes gill and tissue damage, extreme lethargy, convulsions, coma, and death.[86][88] Experiments have shown that the lethal concentration for a variety of fish species ranges from 0.2 to 2.0 mg/l.[88]During winter, when reduced feeds are administered to aquaculture stock, ammonia levels can be higher. Lower ambient temperatures reduce the rate of algal photosynthesis so less ammonia is removed by any algae present. Within an aquaculture environment, especially at large scale, there is no fast-acting remedy to elevated ammonia levels. Prevention rather than correction is recommended to reduce harm to farmed fish[88] and in open water systems, the surrounding environment.
Storage information
Similar to propane, anhydrous ammonia boils below room temperature when at atmospheric pressure. A storage vessel capable of 250 psi (1.7 MPa) is suitable to contain the liquid.[89] Ammonium compounds should never be allowed to come in contact with bases (unless in an intended and contained reaction), as dangerous quantities of ammonia gas could be released.Household use
Solutions of ammonia (5–10% by weight) are used as household cleaners, particularly for glass. These solutions are irritating to the eyes and mucous membranes (respiratory and digestive tracts), and to a lesser extent the skin. Caution should be used that the chemical is never mixed into any liquid containing bleach, as a poisonous gas may result. Mixing with chlorine-containing products or strong oxidants, such as household bleach, can lead to hazardous compounds such as chloramines.[90]Laboratory use of ammonia solutions
Hydrochloric acid sample releasing HCl fumes, which are reacting with
ammonia fumes to produce a white smoke of ammonium chloride.
The hazards of ammonia solutions depend on the concentration: "dilute" ammonia solutions are usually 5–10% by weight (<5 .62="" are="" at="" concentrated="" mol="" nbsp="" prepared="" solutions="" usually="">25% by weight. A 25% (by weight) solution has a density of 0.907 g/cm3, and a solution that has a lower density will be more concentrated. The European Union classification of ammonia solutions is given in the table.
| Concentration by weight (w/w) |
Molarity | Concentration mass/volume (w/v) |
Classification | R-Phrases |
|---|---|---|---|---|
| 5–10% | 2.87–5.62 mol/L | 48.9–95.7 g/L | Irritant (Xi) | R36/37/38 |
| 10–25% | 5.62–13.29 mol/L | 95.7–226.3 g/L | Corrosive (C) | R34 |
| >25% | >13.29 mol/L | >226.3 g/L | Corrosive (C) Dangerous for the environment (N) |
R34, R50 |
Ammonia solutions should not be mixed with halogens, as toxic and/or explosive products are formed. Prolonged contact of ammonia solutions with silver, mercury or iodide salts can also lead to explosive products: such mixtures are often formed in qualitative inorganic analysis, and should be lightly acidified but not concentrated (<6 before="" completed.="" disposal="" is="" once="" p="" test="" the="" v="" w="">
Laboratory use of anhydrous ammonia (gas or liquid)
Anhydrous ammonia is classified as toxic (T) and dangerous for the environment (N). The gas is flammable (autoignition temperature: 651 °C) and can form explosive mixtures with air (16–25%). The permissible exposure limit (PEL) in the United States is 50 ppm (35 mg/m3), while the IDLH concentration is estimated at 300 ppm. Repeated exposure to ammonia lowers the sensitivity to the smell of the gas: normally the odour is detectable at concentrations of less than 50 ppm, but desensitised individuals may not detect it even at concentrations of 100 ppm. Anhydrous ammonia corrodes copper- and zinc-containing alloys, and so brass fittings should not be used for handling the gas. Liquid ammonia can also attack rubber and certain plastics.Ammonia reacts violently with the halogens. Nitrogen triiodide, a primary high explosive, is formed when ammonia comes in contact with iodine. Ammonia causes the explosive polymerisation of ethylene oxide. It also forms explosive fulminating compounds with compounds of gold, silver, mercury, germanium or tellurium, and with stibine. Violent reactions have also been reported with acetaldehyde, hypochlorite solutions, potassium ferricyanide and peroxides.
Synthesis and production
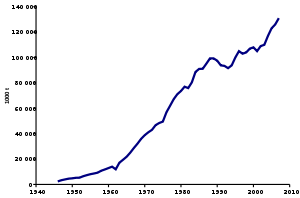
Production trend of ammonia between 1947 and 2007
Because of its many uses, ammonia is one of the most highly produced inorganic chemicals. Dozens of chemical plants worldwide produce ammonia. Consuming more than 1% of all man-made power, ammonia production is a significant component of the world energy budget.[46] The USGS reports global ammonia production in 2014 was 176 million tonnes.[11] China accounted for 32.6% of that (increasingly from coal as part of urea synthesis), followed by Russia at 8.1%, India at 7.6%, and the United States at 6.4%.[11] About 88% of the ammonia produced was used for fertilizing agricultural crops.[11] As of 2012 the global production of ammonia produced from natural gas using the steam reforming process was 72 percent.[91]
Before the start of World War I, most ammonia was obtained by the dry distillation[92] of nitrogenous vegetable and animal waste products, including camel dung, where it was distilled by the reduction of nitrous acid and nitrites with hydrogen; in addition, it was produced by the distillation of coal, and also by the decomposition of ammonium salts by alkaline hydroxides[93] such as quicklime, the salt most generally used being the chloride (sal ammoniac) thus:[15]
- 2 NH4Cl + 2 CaO → CaCl2 + Ca(OH)2 + 2 NH3
Modern ammonia-producing plants depend on industrial hydrogen production to react with atmospheric nitrogen using a magnetite catalyst or over a promoted Fe catalyst under high pressure (100 standard atmospheres (10,000 kPa)) and temperature (450 °C) to form anhydrous liquid ammonia. This step is known as the ammonia synthesis loop (also referred to as the Haber–Bosch process):[95]
- 3 H2 + N2 → 2 NH3
As a sustainable alternative to the relatively inefficient electrolysis, hydrogen can be generated from organic wastes (such as biomass or food-industry waste), using catalytic reforming. This releases hydrogen from carbonaceous substances at only 10–20% of energy used by electrolysis and may lead to hydrogen being produced from municipal wastes at below zero cost (allowing for the tipping fees and efficient catalytic reforming, such as cold-plasma). Catalytic (thermal) reforming is possible in small, distributed (even mobile) plants, to take advantage of low-value, stranded biomass/biowaste or natural gas deposits. Conversion of such wastes into ammonia solves the problem of hydrogen storage, as hydrogen can be released economically from ammonia on-demand, without the need for high-pressure or cryogenic storage.
It is also easier to store ammonia on board vehicles than to store hydrogen, as ammonia is less flammable than petrol or LPG.
For small scale laboratory synthesis, one can heat urea and Ca(OH)2
- (NH2)2CO + Ca(OH)2 → CaCO3 + 2 NH3
Liquid ammonia as a solvent
Liquid ammonia is the best-known and most widely studied nonaqueous ionising solvent. Its most conspicuous property is its ability to dissolve alkali metals to form highly coloured, electrically conductive solutions containing solvated electrons. Apart from these remarkable solutions, much of the chemistry in liquid ammonia can be classified by analogy with related reactions in aqueous solutions. Comparison of the physical properties of NH3 with those of water shows NH3 has the lower melting point, boiling point, density, viscosity, dielectric constant and electrical conductivity; this is due at least in part to the weaker hydrogen bonding in NH3 and because such bonding cannot form cross-linked networks, since each NH3 molecule has only one lone pair of electrons compared with two for each H2O molecule. The ionic self-dissociation constant of liquid NH3 at −50 °C is about 10−33 mol2·l−2.Solubility of salts
| Solubility (g of salt per 100 g liquid NH3) | |
|---|---|
| Ammonium acetate | 253.2 |
| Ammonium nitrate | 389.6 |
| Lithium nitrate | 243.7 |
| Sodium nitrate | 97.6 |
| Potassium nitrate | 10.4 |
| Sodium fluoride | 0.35 |
| Sodium chloride | 157.0 |
| Sodium bromide | 138.0 |
| Sodium iodide | 161.9 |
| Sodium thiocyanate | 205.5 |
Liquid ammonia is an ionising solvent, although less so than water, and dissolves a range of ionic compounds, including many nitrates, nitrites, cyanides, thiocyanates, metal cyclopentadienyl complexes and metal bis(trimethylsilyl)amides.[96] Most ammonium salts are soluble and act as acids in liquid ammonia solutions. The solubility of halide salts increases from fluoride to iodide. A saturated solution of ammonium nitrate contains 0.83 mol solute per mole of ammonia and has a vapour pressure of less than 1 bar even at 25 °C (77 °F).
Solutions of metals
Liquid ammonia will dissolve the alkali metals and other electropositive metals such as magnesium, calcium, strontium, barium, europium and ytterbium. At low concentrations (<0 .06="" a="" and="" are="" blue="" cations="" contain="" deep="" formed:="" href="https://en.wikipedia.org/wiki/Solvated_electron" l="" metal="" mol="" nbsp="" solutions="" these="" title="Solvated electron">solvated electrons, free electrons that are surrounded by a cage of ammonia molecules.
These solutions are very useful as strong reducing agents. At higher concentrations, the solutions are metallic in appearance and in electrical conductivity. At low temperatures, the two types of solution can coexist as immiscible phases.
Redox properties of liquid ammonia
| E° (V, ammonia) | E° (V, water) | |
|---|---|---|
| Li+ + e− ⇌ Li | −2.24 | −3.04 |
| K+ + e− ⇌ K | −1.98 | −2.93 |
| Na+ + e− ⇌ Na | −1.85 | −2.71 |
| Zn2+ + 2e− ⇌ Zn | −0.53 | −0.76 |
| NH4+ + e− ⇌ ½ H2 + NH3 | 0.00 | — |
| Cu2+ + 2e− ⇌ Cu | +0.43 | +0.34 |
| Ag+ + e− ⇌ Ag | +0.83 | +0.80 |
The range of thermodynamic stability of liquid ammonia solutions is very narrow, as the potential for oxidation to dinitrogen, E° (N2 + 6NH4+ + 6e− ⇌ 8NH3), is only +0.04 V. In practice, both oxidation to dinitrogen and reduction to dihydrogen are slow. This is particularly true of reducing solutions: the solutions of the alkali metals mentioned above are stable for several days, slowly decomposing to the metal amide and dihydrogen. Most studies involving liquid ammonia solutions are done in reducing conditions; although oxidation of liquid ammonia is usually slow, there is still a risk of explosion, particularly if transition metal ions are present as possible catalysts.
Ammonia's role in biological systems and human disease
Main symptoms of hyperammonemia (ammonia reaching toxic concentrations).[97]
Ammonia is both a metabolic waste and a metabolic input throughout the biosphere. It is an important source of nitrogen for living systems. Although atmospheric nitrogen abounds (more than 75%), few living creatures are capable of using this atmospheric nitrogen in its diatomic form, N2 gas. Therefore, nitrogen fixation is required for the synthesis of amino acids, which are the building blocks of protein. Some plants rely on ammonia and other nitrogenous wastes incorporated into the soil by decaying matter. Others, such as nitrogen-fixing legumes, benefit from symbiotic relationships with rhizobia that create ammonia from atmospheric nitrogen.[98]
Biosynthesis
In certain organisms, ammonia is produced from atmospheric nitrogen by enzymes called nitrogenases. The overall process is called nitrogen fixation. Intense effort has been directed toward understanding the mechanism of biological nitrogen fixation; the scientific interest in this problem is motivated by the unusual structure of the active site of the enzyme, which consists of an Fe7MoS9 ensemble.[99]Ammonia is also a metabolic product of amino acid deamination catalyzed by enzymes such as glutamate dehydrogenase 1. Ammonia excretion is common in aquatic animals. In humans, it is quickly converted to urea, which is much less toxic, particularly less basic. This urea is a major component of the dry weight of urine. Most reptiles, birds, insects, and snails excrete uric acid solely as nitrogenous waste.
In physiology
Ammonia also plays a role in both normal and abnormal animal physiology. It is biosynthesised through normal amino acid metabolism and is toxic in high concentrations. The liver converts ammonia to urea through a series of reactions known as the urea cycle. Liver dysfunction, such as that seen in cirrhosis, may lead to elevated amounts of ammonia in the blood (hyperammonemia). Likewise, defects in the enzymes responsible for the urea cycle, such as ornithine transcarbamylase, lead to hyperammonemia. Hyperammonemia contributes to the confusion and coma of hepatic encephalopathy, as well as the neurologic disease common in people with urea cycle defects and organic acidurias.[100]Ammonia is important for normal animal acid/base balance. After formation of ammonium from glutamine, α-ketoglutarate may be degraded to produce two molecules of bicarbonate, which are then available as buffers for dietary acids. Ammonium is excreted in the urine, resulting in net acid loss. Ammonia may itself diffuse across the renal tubules, combine with a hydrogen ion, and thus allow for further acid excretion.[101]
Excretion
Ammonium ions are a toxic waste product of metabolism in animals. In fish and aquatic invertebrates, it is excreted directly into the water. In mammals, sharks, and amphibians, it is converted in the urea cycle to urea, because it is less toxic and can be stored more efficiently. In birds, reptiles, and terrestrial snails, metabolic ammonium is converted into uric acid, which is solid, and can therefore be excreted with minimal water loss.[102]In astronomy
Ammonia occurs in the atmospheres of the outer gas planets such as Jupiter (0.026% ammonia) and Saturn (0.012% ammonia).
Ammonia has been detected in the atmospheres of the gas giant planets, including Jupiter, along with other gases like methane, hydrogen, and helium. The interior of Saturn may include frozen crystals of ammonia.[103] It is naturally found on Deimos and Phobos – the two moons of Mars.
Interstellar space
Ammonia was first detected in interstellar space in 1968, based on microwave emissions from the direction of the galactic core.[104] This was the first polyatomic molecule to be so detected. The sensitivity of the molecule to a broad range of excitations and the ease with which it can be observed in a number of regions has made ammonia one of the most important molecules for studies of molecular clouds.[105] The relative intensity of the ammonia lines can be used to measure the temperature of the emitting medium.The following isotopic species of ammonia have been detected:
- NH3, 15NH3, NH2D, NHD2, and ND3
Since its interstellar discovery, NH3 has proved to be an invaluable spectroscopic tool in the study of the interstellar medium. With a large number of transitions sensitive to a wide range of excitation conditions, NH3 has been widely astronomically detected – its detection has been reported in hundreds of journal articles.
The study of interstellar ammonia has been important to a number of areas of research in the last few decades. Some of these are delineated below and primarily involve using ammonia as an interstellar thermometer.
Interstellar formation mechanisms
Ball-and-stick model of the diamminesilver(I) cation, [Ag(NH3)2]+
The interstellar abundance for ammonia has been measured for a variety of environments. The [NH3]/[H2] ratio has been estimated to range from 10−7 in small dark clouds[107] up to 10−5 in the dense core of the Orion Molecular Cloud Complex.[108] Although a total of 18 total production routes have been proposed,[109] the principal formation mechanism for interstellar NH3 is the reaction:
- NH4+ + e− → NH3 + H·
 . For the primary formation reaction, a = 1.05×10−6 and B = −0.47. Assuming an NH4+ abundance of 3×10−7 and an electron abundance of 10−7 typical of molecular clouds, the formation will proceed at a rate of 1.6×10−9 cm−3s−1 in a molecular cloud of total density 105 cm−3.[111]
. For the primary formation reaction, a = 1.05×10−6 and B = −0.47. Assuming an NH4+ abundance of 3×10−7 and an electron abundance of 10−7 typical of molecular clouds, the formation will proceed at a rate of 1.6×10−9 cm−3s−1 in a molecular cloud of total density 105 cm−3.[111]All other proposed formation reactions have rate constants of between 2 and 13 orders of magnitude smaller, making their contribution to the abundance of ammonia relatively insignificant.[112] As an example of the minor contribution other formation reactions play, the reaction:
- H2 + NH2 → NH3 + H
Some of the other possible formation reactions are:
- H− + NH4+ → NH3 + H2
- PNH3+ + e− → P + NH3
Interstellar destruction mechanisms
There are 113 total proposed reactions leading to the destruction of NH3. Of these, 39 were tabulated in extensive tables of the chemistry among C, N, and O compounds.[113] A review of interstellar ammonia cites the following reactions as the principal dissociation mechanisms:[105]-
NH3 + H3+ → NH4+ + H2(1)
-
NH3 + HCO+ → NH4+ + CO(2)
Single antenna detections
Radio observations of NH3 from the Effelsberg 100-m Radio Telescope reveal that the ammonia line is separated into two components – a background ridge and an unresolved core. The background corresponds well with the locations previously detected CO.[117] The 25 m Chilbolton telescope in England detected radio signatures of ammonia in H II regions, HNH2O masers, H-H objects, and other objects associated with star formation. A comparison of emission line widths indicates that turbulent or systematic velocities do not increase in the central cores of molecular clouds.[118]Microwave radiation from ammonia was observed in several galactic objects including W3(OH), Orion A, W43, W51, and five sources in the galactic centre. The high detection rate indicates that this is a common molecule in the interstellar medium and that high-density regions are common in the galaxy.[119]
Interferometric studies
VLA observations of NH3 in seven regions with high-velocity gaseous outflows revealed condensations of less than 0.1 pc in L1551, S140, and Cepheus A. Three individual condensations were detected in Cepheus A, one of them with a highly elongated shape. They may play an important role in creating the bipolar outflow in the region.[120]Extragalactic ammonia was imaged using the VLA in IC 342. The hot gas has temperatures above 70 K, which was inferred from ammonia line ratios and appears to be closely associated with the innermost portions of the nuclear bar seen in CO.[121] NH3 was also monitored by VLA toward a sample of four galactic ultracompact HII regions: G9.62+0.19, G10.47+0.03, G29.96-0.02, and G31.41+0.31. Based upon temperature and density diagnostics, it is concluded that in general such clumps are probably the sites of massive star formation in an early evolutionary phase prior to the development of an ultracompact HII region.[122]
Infrared detections
Absorption at 2.97 micrometres due to solid ammonia was recorded from interstellar grains in the Becklin-Neugebauer Object and probably in NGC 2264-IR as well. This detection helped explain the physical shape of previously poorly understood and related ice absorption lines.[123]A spectrum of the disk of Jupiter was obtained from the Kuiper Airborne Observatory, covering the 100 to 300 cm−1 spectral range. Analysis of the spectrum provides information on global mean properties of ammonia gas and an ammonia ice haze.[124]
A total of 149 dark cloud positions were surveyed for evidence of 'dense cores' by using the (J,K) = (1,1) rotating inversion line of NH3. In general, the cores are not spherically shaped, with aspect ratios ranging from 1.1 to 4.4. It is also found that cores with stars have broader lines than cores without stars.[125]
Ammonia has been detected in the Draco Nebula and in one or possibly two molecular clouds, which are associated with the high-latitude galactic infrared cirrus. The finding is significant because they may represent the birthplaces for the Population I metallicity B-type stars in the galactic halo that could have been borne in the galactic disk.[126]