| Referred pain | |
|---|---|
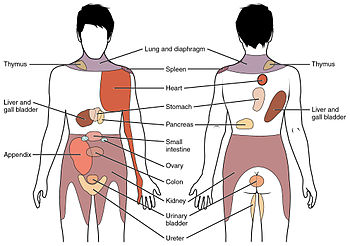
Conscious
perception of visceral sensations map to specific regions of the body,
as shown in this chart. Some sensations are felt locally, whereas others
are perceived as affecting areas that are quite distant from the
involved organ.
| |
| Identifiers | |
| MeSH | D053591 |
Referred pain, also called reflective pain, is pain perceived at a location other than the site of the painful stimulus. An example is the case of angina pectoris brought on by a myocardial infarction (heart attack), where pain is often felt in the neck, shoulders, and back rather than in the thorax (chest), the site of the injury. The International Association for the Study of Pain has not officially defined the term; hence several authors have defined it differently.
Radiating pain is slightly different from referred pain; for example, the pain related to a myocardial infarction could either be referred or radiating pain from the chest. Referred pain is when the pain is located away from or adjacent to the organ involved; for instance, when a person has pain only in their jaw or left arm, but not in the chest. Referred pain has been described since the late 1880s. Despite an increasing amount of literature on the subject, the biological mechanism of referred pain is unknown, although there are several hypotheses.
Characteristics
- The size of referred pain is related to the intensity and duration of ongoing/evoked pain.
- Temporal summation is a potent mechanism for generation of referred muscle pain.
- Central hyperexcitability is important for the extent of referred pain.
- Patients with chronic musculoskeletal pains have enlarged referred pain areas to experimental stimuli. The proximal spread of referred muscle pain is seen in patients with chronic musculoskeletal pain and very seldom is it seen in healthy individuals.
- Modality-specific somatosensory changes occur in referred areas, which emphasize the importance of using a multimodal sensory test regime for assessment.
- Referred pain is often experienced on the same side of the body as the source, but not always.
Mechanism
There
are several proposed mechanisms for referred pain. Currently there is
no definitive consensus regarding which is correct. The cardiac general
visceral sensory pain fibers follow the sympathetics back to the spinal
cord and have their cell bodies located in thoracic dorsal root ganglia
1-4(5).
As a general rule, in the thorax and abdomen, general visceral afferent
(GVA) pain fibers follow sympathetic fibers back to the same spinal cord
segments that gave rise to the preganglionic sympathetic fibers.
The central nervous system (CNS) perceives pain from the heart as coming
from the somatic portion of the body supplied by the thoracic spinal
cord segments 1-4(5). Classically the pain associated with a myocardial
infarction is located in the mid or left side of the chest where the
heart is actually located. The pain can radiate to the left side of the
jaw and into the left arm. Myocardial infarction can rarely present as
referred pain and this usually occurs in people with diabetes or older age. Also, the dermatomes
of this region of the body wall and upper limb have their neuronal cell
bodies in the same dorsal root ganglia (T1-5) and synapse in the same
second order neurons in the spinal cord segments (T1-5) as the general
visceral sensory fibers from the heart. The CNS does not clearly discern
whether the pain is coming from the body wall or from the viscera, but
it perceives the pain as coming from somewhere on the body wall, i.e.
substernal pain, left arm/hand pain, jaw pain.
Convergent-projection
This
represents one of the earliest theories on the subject of referred
pain. It is based on the work of W.A. Sturge and J. Ross from 1888 and
later TC Ruch in 1961. Convergent projection proposes that afferent
nerve fibers from tissues converge onto the same spinal neuron, and
explains why referred pain is believed to be segmented in much the same
way as the spinal cord. Additionally, experimental evidence shows that
when local pain (pain at the site of stimulation) is intensified the
referred pain is intensified as well.
Criticism of this model arises from its inability to explain why
there is a delay between the onset of referred pain after local pain
stimulation. Experimental evidence also shows that referred pain is
often unidirectional. For example, stimulated local pain in the anterior
tibial muscle causes referred pain in the ventral portion of the ankle;
however referred pain moving in the opposite direction has not been
shown experimentally. Lastly, the threshold for the local pain
stimulation and the referred pain stimulation are different, but
according to this model they should both be the same.
Convergence-facilitation
Convergence
facilitation was conceived in 1893 by J MacKenzie based on the ideas of
Sturge and Ross. He believed that the internal organs were insensitive
to stimuli. Furthermore, he believed that non-nociceptive afferent
inputs to the spinal cord
created what he termed "an irritable focus". This focus caused some
stimuli to be perceived as referred pain. However, his ideas did not
gain widespread acceptance from critics due to its dismissal of visceral
pain.
Recently this idea has regained some credibility under a new term, central sensitization.
Central sensitization occurs when neurons in the spinal cord's dorsal
horn or brainstem become more responsive after repeated stimulation by
peripheral neurons, so that weaker signals can trigger them. The delay
in appearance of referred pain shown in laboratory experiments can be
explained due to the time required to create the central sensitization.
Axon-reflex
Axon reflex suggests that the afferent fiber is bifurcated before connecting to the dorsal horn.
Bifurcated fibers do exist in muscle, skin, and intervertebral discs.
Yet these particular neurons are rare and are not representative of the
whole body. Axon-Reflex also does not explain the time delay before the
appearance of referred pain, threshold differences for stimulating local
and referred pain, and somatosensory sensibility changes in the area of
referred pain.
Hyperexcitability
Hyperexcitability
hypothesizes that referred pain has no central mechanism. However, it
does say that there is one central characteristic that predominates.
Experiments involving noxious stimuli and recordings from the dorsal
horn of animals revealed that referred pain sensations began minutes
after muscle stimulation. Pain was felt in a receptive field that was
some distance away from the original receptive field. According to
hyperexcitability, new receptive fields are created as a result of the
opening of latent convergent afferent fibers in the dorsal horn. This
signal could then be perceived as referred pain.
Several characteristics are in line with this mechanism of
referred pain, such as dependency on stimulus and the time delay in the
appearance of referred pain as compared to local pain. However, the
appearance of new receptive fields, which is interpreted to be referred
pain, conflicts with the majority of experimental evidence from studies
including studies of healthy individuals. Furthermore, referred pain
generally appears within seconds in humans as opposed to minutes in
animal models. Some scientists attribute this to a mechanism or
influence downstream in the supraspinal pathways. Neuroimaging
techniques such as PET scans or fMRI may visualize the underlying neural processing pathways responsible in future testing.
Thalamic-convergence
Thalamic
convergence suggests that referred pain is perceived as such due to the
summation of neural inputs in the brain, as opposed to the spinal cord,
from the injured area and the referred area. Experimental evidence on
thalamic convergence is lacking. However, pain studies performed on
monkeys revealed convergence of several pathways upon separate cortical
and subcortical neurons.
Examples
| Location | Description |
|---|---|
| Upper chest/left limb | Myocardial ischaemia (the loss of blood flow to a part of the heart muscle tissue) is possibly the best known example of referred pain; the sensation can occur in the upper chest as a restricted feeling, or as an ache in the left shoulder, arm or even hand. |
| Head | "Ice-cream headache" or "brain freeze" is another example of referred pain, in which the vagus nerve or the trigeminal nerve in the throat and the palate, respectively, transmit pain signals, because of the rapid cooling and rewarming of the capillaries in the sinuses.[4] |
| General | Phantom limb pain, a type of referred pain, is the sensation of pain from a limb that has been lost or from which a person no longer receives physical signals. It is an experience almost universally reported by amputees and quadriplegics. |
| Right tip of scapula | Liver, gallbladder[citation needed] |
| Left shoulder | Thoracic diaphragm, Spleen (Kehr's sign), lung |
| Back | Pancreas |
| Palm of Hand | Palmaris longus A problem originating in the forearm might be felt in the palm, and not in the forearm. |
Laboratory testing methods
Pain
is studied in a laboratory setting due to the greater amount of control
that can be exerted. For example, the modality, intensity, and timing
of painful stimuli can be controlled with much more precision. Within
this setting there are two main ways that referred pain is studied.
Algogenic substances
In recent years several different chemicals have been used to induce referred pain including bradykinin, substance P, capsaicin, and serotonin. However, before any of these substances became widespread in their use a solution of hypertonic saline
was used instead. Through various experiments it was determined that
there were multiple factors that correlated with saline administration
such as infusion rate, saline concentration, pressure, and amount of
saline used. The mechanism by which the saline induces a local and
referred pain pair is unknown. Some researchers have commented that it
could be due to osmotic differences, however that is not verified.
Using electrical stimulation
Intramuscular
electrical stimulation (IMES) of muscle tissue has been used in various
experimental and clinical settings. The advantage to using an IMES
system over a standard such as hypertonic saline is that IMES can be
turned on and off. This allows the researcher to exert a much higher
degree of control and precision in terms of the stimulus and the
measurement of the response. The method is easier to carry out than the
injection method as it does not require special training in how it
should be used. The frequency of the electrical pulse can also be
controlled. For most studies a frequency of about 10 Hz is needed to
stimulate both local and referred pain.
Using this method it has been observed that significantly higher
stimulus strength is needed to obtain referred pain relative to the
local pain. There is also a strong correlation between the stimulus
intensity and the intensity of referred and local pain. It is also
believed that this method causes a larger recruitment of nociceptor
units resulting in a spatial summation. This spatial summation results
in a much larger barrage of signals to the dorsal horn and brainstem neurons.
Use in clinical diagnosis and treatments
Referred
pain can be indicative of nerve damage. A case study done on a
63-year-old man with an injury sustained during his childhood developed
referred pain symptoms after his face or back was touched. After even a
light touch, there was a shooting pain in his arm. The study concluded
that his pain was possibly due to a neural reorganization which
sensitized regions of his face and back after the nerve damage occurred.
It is mentioned that this case is very similar to what phantom limb
syndrome patients suffer. This conclusion was based on experimental
evidence gathered by V. Ramachandran in 1993, with the difference being
that the arm that is in pain is still attached to the body.
Orthopedic diagnosis
From
the above examples one can see why understanding of referred pain can
lead to better diagnoses of various conditions and diseases. In 1981
physiotherapist Robin McKenzie described what he termed centralization.
He concluded that centralization occurs when referred pain moves from a
distal to a more proximal location. Observations in support of this
idea were seen when patients would bend backward and forward during an
examination.
Studies have reported that the majority of patients that
experienced centralization were able to avoid spinal surgery through
isolating the area of local pain. However, the patients who did not
experience centralization had to undergo surgery to diagnose and correct
the problems. As a result of this study there has been a further
research into the elimination of referred pain through certain body
movements.
One example of this is referred pain in the calf. McKenzie showed
that the referred pain would move closer to the spine when the patient
bent backwards in full extension a few times. More importantly, the
referred pain would dissipate even after the movements were stopped.
General diagnosis
As with myocardial ischaemia
referred pain in a certain portion of the body can lead to a diagnosis
of the correct local center. Somatic mapping of referred pain and the
corresponding local centers has led to various topographic maps being
produced to aid in pinpointing the location of pain based on the
referred areas. For example, local pain stimulated in the esophagus is
capable of producing referred pain in the upper abdomen, the oblique
muscles, and the throat. Local pain in the prostate can radiate referred
pain to the abdomen, lower back, and calf muscles. Kidney stones
can cause visceral pain in the ureter as the stone is slowly passed
into the excretory system. This can cause immense referred pain in the
lower abdominal wall.
Further, recent research has found that ketamine, a sedative, is capable of blocking referred pain. The study was conducted on patients suffering from fibromyalgia,
a disease characterized by joint and muscle pain and fatigue. These
patients were looked at specifically due to their increased sensitivity
to nociceptive stimuli. Furthermore, referred pain appears in a
different pattern in fibromyalgic patients than non-fibromyalgic
patients. Often this difference manifests as a difference in terms of
the area that the referred pain is found (distal vs. proximal) as
compared to the local pain. The area is also much more exaggerated owing
to the increased sensitivity.
