From Wikipedia, the free encyclopedia
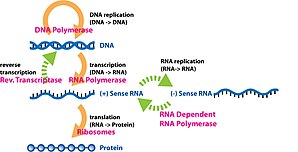
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. These products are often proteins, but in non-protein-coding genes such as transfer RNA (tRNA) and small nuclear RNA (snRNA), the product is a functional non-coding RNA. Gene expression is summarized in the central dogma of molecular biology first formulated by Francis Crick in 1958, further developed in his 1970 article, and expanded by the subsequent discoveries of reverse transcription and RNA replication.
The process of gene expression is used by all known life—eukaryotes (including multicellular organisms), prokaryotes (bacteria and archaea), and utilized by viruses—to generate the macromolecular machinery for life.
In genetics, gene expression is the most fundamental level at which the genotype gives rise to the phenotype, i.e. observable trait. The genetic information stored in DNA
represents the genotype, whereas the phenotype results from the
"interpretation" of that information. Such phenotypes are often
expressed by the synthesis of proteins that control the organism's
structure and development, or that act as enzymes catalyzing specific metabolic pathways.
All steps in the gene expression process may be modulated (regulated), including the transcription, RNA splicing, translation, and post-translational modification of a protein. Regulation of gene expression
gives control over the timing, location, and amount of a given gene
product (protein or ncRNA) present in a cell and can have a profound
effect on the cellular structure and function. Regulation of gene
expression is the basis for cellular differentiation, development, morphogenesis and the versatility and adaptability of any organism. Gene regulation may therefore serve as a substrate for evolutionary change.
Mechanism
Transcription
The
process of transcription is carried out by RNA polymerase (RNAP), which
uses DNA (black) as a template and produces RNA (blue).
The production of a RNA copy from a DNA strand is called transcription, and is performed by RNA polymerases, which add one ribonucleotide at a time to a growing RNA strand as per the complementarity law of the nucleotide bases. This RNA is complementary to the template 3′ → 5′ DNA strand, with the exception that thymines (T) are replaced with uracils (U) in the RNA.
In prokaryotes, transcription is carried out by a single type of RNA polymerase, which needs to bind a DNA sequence called a Pribnow box with the help of the sigma factor
protein (σ factor) to start transcription. In eukaryotes, transcription
is performed in the nucleus by three types of RNA polymerases, each of
which needs a special DNA sequence called the promoter and a set of DNA-binding proteins—transcription factors—to initiate the process (see regulation of transcription below). RNA polymerase I is responsible for transcription of ribosomal RNA (rRNA) genes. RNA polymerase II (Pol II) transcribes all protein-coding genes but also some non-coding RNAs (e.g., snRNAs, snoRNAs or long non-coding RNAs). RNA polymerase III transcribes 5S rRNA, transfer RNA (tRNA) genes, and some small non-coding RNAs (e.g., 7SK). Transcription ends when the polymerase encounters a sequence called the terminator.
mRNA processing
While transcription of prokaryotic protein-coding genes creates messenger RNA (mRNA) that is ready for translation into protein, transcription of eukaryotic genes leaves a primary transcript of RNA (pre-RNA),
which first has to undergo a series of modifications to become a mature
RNA. Types and steps involved in the maturation processes vary between
coding and non-coding preRNAs; i.e. even though preRNA molecules for both mRNA and tRNA undergo splicing, the steps and machinery involved are different. The processing of non-coding RNA is described below (non-coring RNA maturation).
The processing of premRNA include 5′ capping, which is set of enzymatic reactions that add 7-methylguanosine (m7G) to the 5′ end of pre-mRNA and thus protect the RNA from degradation by exonucleases. The m7G cap is then bound by cap binding complex heterodimer (CBC20/CBC80), which aids in mRNA export to cytoplasm and also protect the RNA from decapping.
Another modification is 3′ cleavage and polyadenylation.
They occur if polyadenylation signal sequence (5′- AAUAAA-3′) is present
in pre-mRNA, which is usually between protein-coding sequence and
terminator. The pre-mRNA is first cleaved and then a series of ~200
adenines (A) are added to form poly(A) tail, which protects the RNA from
degradation. The poly(A) tail is bound by multiple poly(A)-binding proteins (PABPs)
necessary for mRNA export and translation re-initiation. In the inverse
process of deadenylation, poly(A) tails are shortened by the CCR4-Not 3′-5′ exonuclease, which often leads to full transcript decay.
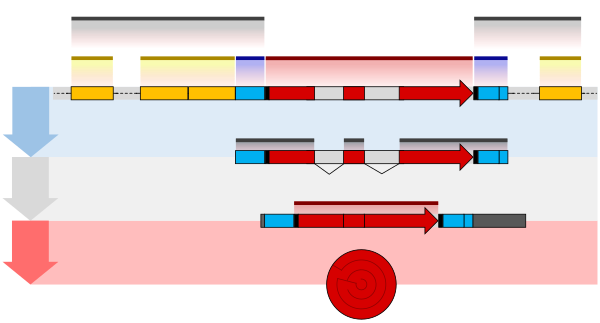
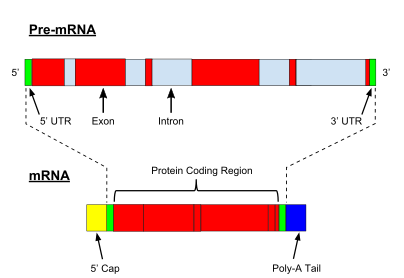
Illustration
of exons and introns in pre-mRNA and the formation of mature mRNA by
splicing. The UTRs (in green) are non-coding parts of exons at the ends
of the mRNA.
A very important modification of eukaryotic pre-mRNA is RNA splicing. The majority of eukaryotic pre-mRNAs consist of alternating segments called exons and introns. During the process of splicing, an RNA-protein catalytical complex known as spliceosome
catalyzes two transesterification reactions, which remove an intron and
release it in form of lariat structure, and then splice neighbouring
exons together. In certain cases, some introns or exons can be either
removed or retained in mature mRNA. This so-called alternative splicing
creates series of different transcripts originating from a single gene.
Because these transcripts can be potentially translated into different
proteins, splicing extends the complexity of eukaryotic gene expression
and the size of a species proteome.
Extensive RNA processing may be an evolutionary advantage
made possible by the nucleus of eukaryotes. In prokaryotes,
transcription and translation happen together, whilst in eukaryotes, the
nuclear membrane separates the two processes, giving time for RNA processing to occur.
Non-coding RNA maturation
In most organisms non-coding genes (ncRNA)
are transcribed as precursors that undergo further processing. In the
case of ribosomal RNAs (rRNA), they are often transcribed as a pre-rRNA
that contains one or more rRNAs. The pre-rRNA is cleaved and modified
(2′-O-methylation and pseudouridine
formation) at specific sites by approximately 150 different small
nucleolus-restricted RNA species, called snoRNAs. SnoRNAs associate with
proteins, forming snoRNPs. While snoRNA part basepair with the target
RNA and thus position the modification at a precise site, the protein
part performs the catalytical reaction. In eukaryotes, in particular a
snoRNP called RNase, MRP cleaves the 45S pre-rRNA into the 28S, 5.8S,
and 18S rRNAs. The rRNA and RNA processing factors form large aggregates
called the nucleolus.
In the case of transfer RNA (tRNA), for example, the 5′ sequence is removed by RNase P, whereas the 3′ end is removed by the tRNase Z enzyme and the non-templated 3′ CCA tail is added by a nucleotidyl transferase. In the case of micro RNA (miRNA),
miRNAs are first transcribed as primary transcripts or pri-miRNA with a
cap and poly-A tail and processed to short, 70-nucleotide stem-loop
structures known as pre-miRNA in the cell nucleus by the enzymes Drosha and Pasha. After being exported, it is then processed to mature miRNAs in the cytoplasm by interaction with the endonuclease Dicer, which also initiates the formation of the RNA-induced silencing complex (RISC), composed of the Argonaute protein.
Even snRNAs and snoRNAs themselves undergo series of modification
before they become part of functional RNP complex. This is done either
in the nucleoplasm or in the specialized compartments called Cajal bodies. Their bases are methylated or pseudouridinilated by a group of small Cajal body-specific RNAs (scaRNAs), which are structurally similar to snoRNAs.
RNA export
In eukaryotes most mature RNA must be exported to the cytoplasm from the nucleus. While some RNAs function in the nucleus, many RNAs are transported through the nuclear pores and into the cytosol.
Export of RNAs requires association with specific proteins known as
exportins. Specific exportin molecules are responsible for the export of
a given RNA type. mRNA transport also requires the correct association
with Exon Junction Complex
(EJC), which ensures that correct processing of the mRNA is completed
before export. In some cases RNAs are additionally transported to a
specific part of the cytoplasm, such as a synapse; they are then towed by motor proteins that bind through linker proteins to specific sequences (called "zipcodes") on the RNA.
Translation
For some RNA (non-coding RNA) the mature RNA is the final gene product.
In the case of messenger RNA (mRNA) the RNA is an information carrier
coding for the synthesis of one or more proteins. mRNA carrying a single
protein sequence (common in eukaryotes) is monocistronic whilst mRNA carrying multiple protein sequences (common in prokaryotes) is known as polycistronic.
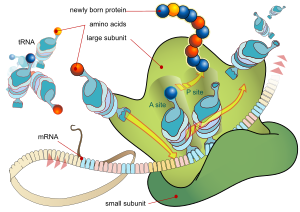
During
the translation, tRNA charged with amino acid enters the ribosome and
aligns with the correct mRNA triplet. Ribosome then adds amino acid to
growing protein chain.
Every mRNA consists of three parts: a 5′ untranslated region (5′UTR),
a protein-coding region or open reading frame (ORF), and a 3′
untranslated region (3′UTR). The coding region carries information for
protein synthesis encoded by the genetic code to form triplets. Each triplet of nucleotides of the coding region is called a codon
and corresponds to a binding site complementary to an anticodon triplet
in transfer RNA. Transfer RNAs with the same anticodon sequence always
carry an identical type of amino acid. Amino acids are then chained together by the ribosome
according to the order of triplets in the coding region. The ribosome
helps transfer RNA to bind to messenger RNA and takes the amino acid
from each transfer RNA and makes a structure-less protein out of it. Each mRNA molecule is translated into many protein molecules, on average ~2800 in mammals.
In prokaryotes translation generally occurs at the point of
transcription (co-transcriptionally), often using a messenger RNA that
is still in the process of being created. In eukaryotes translation can
occur in a variety of regions of the cell depending on where the protein
being written is supposed to be. Major locations are the cytoplasm for soluble cytoplasmic proteins and the membrane of the endoplasmic reticulum for proteins that are for export from the cell or insertion into a cell membrane.
Proteins that are supposed to be expressed at the endoplasmic reticulum
are recognised part-way through the translation process. This is
governed by the signal recognition particle—a protein that binds to the ribosome and directs it to the endoplasmic reticulum when it finds a signal peptide on the growing (nascent) amino acid chain.
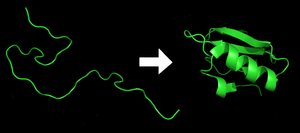
Folding
Protein before (left) and after (right) folding
Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA into a linear chain of amino acids.
This polypeptide lacks any developed three-dimensional structure (the
left hand side of the neighboring figure). The polypeptide then folds
into its characteristic and functional three-dimensional structure from a random coil.
Amino acids interact with each other to produce a well-defined
three-dimensional structure, the folded protein (the right hand side of
the figure) known as the native state. The resulting three-dimensional structure is determined by the amino acid sequence (Anfinsen's dogma).
The correct three-dimensional structure is essential to function, although some parts of functional proteins may remain unfolded. Failure to fold into the intended shape usually produces inactive proteins with different properties including toxic prions. Several neurodegenerative and other diseases are believed to result from the accumulation of misfolded proteins. Many allergies are caused by the folding of the proteins, for the immune system does not produce antibodies for certain protein structures.
Enzymes called chaperones assist the newly formed protein to attain (fold into) the 3-dimensional structure it needs to function. Similarly, RNA chaperones help RNAs attain their functional shapes. Assisting protein folding is one of the main roles of the endoplasmic reticulum in eukaryotes.
Translocation
Secretory
proteins of eukaryotes or prokaryotes must be translocated to enter the
secretory pathway. Newly synthesized proteins are directed to the
eukaryotic Sec61 or prokaryotic SecYEG translocation channel by signal peptides. The efficiency of protein secretion in eukaryotes is very dependent on the signal peptide which has been used.
Protein transport
Many proteins are destined for other parts of the cell than the cytosol and a wide range of signalling sequences or (signal peptides)
are used to direct proteins to where they are supposed to be. In
prokaryotes this is normally a simple process due to limited
compartmentalisation of the cell. However, in eukaryotes there is a
great variety of different targeting processes to ensure the protein
arrives at the correct organelle.
Not all proteins remain within the cell and many are exported, for example, digestive enzymes, hormones and extracellular matrix
proteins. In eukaryotes the export pathway is well developed and the
main mechanism for the export of these proteins is translocation to the
endoplasmic reticulum, followed by transport via the Golgi apparatus.
Regulation of gene expression
Regulation of gene expression is the control of the amount and timing
of appearance of the functional product of a gene. Control of
expression is vital to allow a cell to produce the gene products it
needs when it needs them; in turn, this gives cells the flexibility to
adapt to a variable environment, external signals, damage to the cell,
and other stimuli. More generally, gene regulation gives the cell
control over all structure and function, and is the basis for cellular differentiation, morphogenesis and the versatility and adaptability of any organism.
Numerous terms are used to describe types of genes depending on how they are regulated; these include:
- A constitutive gene is a gene that is transcribed continually as opposed to a facultative gene, which is only transcribed when needed.
- A housekeeping gene
is a gene that is required to maintain basic cellular function and so
is typically expressed in all cell types of an organism. Examples
include actin, GAPDH and ubiquitin.
Some housekeeping genes are transcribed at a relatively constant rate
and these genes can be used as a reference point in experiments to
measure the expression rates of other genes.
- A facultative gene is a gene only transcribed when needed as opposed to a constitutive gene.
- An inducible gene is a gene whose expression is either responsive to environmental change or dependent on the position in the cell cycle.
Any step of gene expression may be modulated, from the DNA-RNA transcription step to post-translational modification
of a protein. The stability of the final gene product, whether it is
RNA or protein, also contributes to the expression level of the gene—an
unstable product results in a low expression level. In general gene
expression is regulated through changes in the number and type of interactions between molecules that collectively influence transcription of DNA and translation of RNA.
Some simple examples of where gene expression is important are:
Transcriptional regulation
When
lactose is present in a prokaryote, it acts as an inducer and
inactivates the repressor so that the genes for lactose metabolism can
be transcribed.
Regulation of transcription can be broken down into three main routes
of influence; genetic (direct interaction of a control factor with the
gene), modulation interaction of a control factor with the transcription
machinery and epigenetic (non-sequence changes in DNA structure that
influence transcription).
Direct interaction with DNA is the simplest and the most direct
method by which a protein changes transcription levels. Genes often have
several protein binding sites around the coding region with the
specific function of regulating transcription. There are many classes of
regulatory DNA binding sites known as enhancers, insulators and silencers. The mechanisms for regulating transcription are varied, from blocking key binding sites on the DNA for RNA polymerase to acting as an activator and promoting transcription by assisting RNA polymerase binding.
The activity of transcription factors is further modulated by
intracellular signals causing protein post-translational modification
including phosphorylated, acetylated, or glycosylated.
These changes influence a transcription factor's ability to bind,
directly or indirectly, to promoter DNA, to recruit RNA polymerase, or
to favor elongation of a newly synthesized RNA molecule.
The nuclear membrane in eukaryotes allows further regulation of
transcription factors by the duration of their presence in the nucleus,
which is regulated by reversible changes in their structure and by
binding of other proteins. Environmental stimuli or endocrine signals may cause modification of regulatory proteins eliciting cascades of intracellular signals, which result in regulation of gene expression.
More recently it has become apparent that there is a significant
influence of non-DNA-sequence specific effects on transcription. These
effects are referred to as epigenetic
and involve the higher order structure of DNA, non-sequence specific
DNA binding proteins and chemical modification of DNA. In general
epigenetic effects alter the accessibility of DNA to proteins and so
modulate transcription.
In eukaryotes, DNA is organized in form of
nucleosomes. Note how the DNA (blue and green) is tightly wrapped around the protein core made of
histone octamer (ribbon coils), restricting access to the DNA. From
PDB: 1KX5.
In eukaryotes the structure of chromatin, controlled by the histone code, regulates access to DNA with significant impacts on the expression of genes in euchromatin and heterochromatin areas.
Enhancers, transcription factors, mediator complex and DNA loops in mammalian transcription
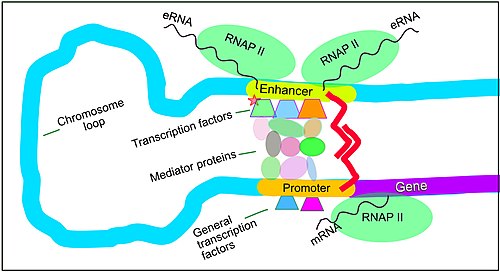
Regulation of transcription in mammals. An active
enhancer regulatory region is enabled to interact with the
promoter region of its target
gene by formation of a chromosome loop. This can initiate
messenger RNA (mRNA) synthesis by
RNA polymerase II (RNAP II) bound to the promoter at the
transcription start site
of the gene. The loop is stabilized by one architectural protein
anchored to the enhancer and one anchored to the promoter and these
proteins are joined to form a dimer (red zigzags). Specific regulatory
transcription factors
bind to DNA sequence motifs on the enhancer. General transcription
factors bind to the promoter. When a transcription factor is activated
by a signal (here indicated as
phosphorylation
shown by a small red star on a transcription factor on the enhancer)
the enhancer is activated and can now activate its target promoter. The
active enhancer is transcribed on each strand of DNA in opposite
directions by bound RNAP IIs. Mediator (a complex consisting of about 26
proteins in an interacting structure) communicates regulatory signals
from the enhancer DNA-bound transcription factors to the promoter.
Gene expression in mammals is regulated by many cis-regulatory elements, including core promoters and promoter-proximal elements that are located near the transcription start sites of genes, upstream on the DNA (towards the 5' region of the sense strand).
Other important cis-regulatory modules are localized in DNA regions
that are distant from the transcription start sites. These include enhancers, silencers, insulators and tethering elements. Enhancers and their associated transcription factors have a leading role in the regulation of gene expression.
Enhancers
are genome regions that regulate genes. Enhancers control
cell-type-specific gene expression programs, most often by looping
through long distances to come in physical proximity with the promoters
of their target genes.
Multiple enhancers, each often tens or hundred of thousands of
nucleotides distant from their target genes, loop to their target gene
promoters and coordinate with each other to control gene expression.
The illustration shows an enhancer looping around to come into
proximity with the promoter of a target gene. The loop is stabilized by a
dimer of a connector protein (e.g. dimer of CTCF or YY1).
One member of the dimer is anchored to its binding motif on the
enhancer and the other member is anchored to its binding motif on the
promoter (represented by the red zigzags in the illustration). Several cell function-specific transcription factors (among the about 1,600 transcription factors in a human cell) generally bind to specific motifs on an enhancer.
A small combination of these enhancer-bound transcription factors, when
brought close to a promoter by a DNA loop, govern transcription level
of the target gene. Mediator (a complex usually consisting of about 26
proteins in an interacting structure) communicates regulatory signals
from enhancer DNA-bound transcription factors directly to the RNA
polymerase II (pol II) enzyme bound to the promoter.
Enhancers, when active, are generally transcribed from both
strands of DNA with RNA polymerases acting in two different directions,
producing two eRNAs as illustrated in the figure.
An inactive enhancer may be bound by an inactive transcription factor.
Phosphorylation of the transcription factor may activate it and that
activated transcription factor may then activate the enhancer to which
it is bound (see small red star representing phosphorylation of
transcription factor bound to enhancer in the illustration). An activated enhancer begins transcription of its RNA before activating transcription of messenger RNA from its target gene.
DNA methylation and demethylation in transcriptional regulation
DNA methylation is the addition of a
methyl group to the DNA that happens at
cytosine.
The image shows a cytosine single ring base and a methyl group added on
to the 5 carbon. In mammals, DNA methylation occurs almost exclusively
at a cytosine that is followed by a
guanine.
DNA methylation is a widespread mechanism for epigenetic influence on gene expression and is seen in bacteria and eukaryotes
and has roles in heritable transcription silencing and transcription
regulation. Methylation most often occurs on a cytosine (see Figure).
Methylation of cytosine primarily occurs in dinucleotide sequences where
a cytosine is followed by a guanine, a CpG site. The number of CpG sites in the human genome is about 28 million. Depending on the type of cell, about 70% of the CpG sites have a methylated cytosine.
Methylation of cytosine in DNA has a major role in regulating
gene expression. Methylation of CpGs in a promoter region of a gene
usually represses gene transcription while methylation of CpGs in the body of a gene increases expression. TET enzymes play a central role in demethylation of methylated cytosines. Demethylation of CpGs in a gene promoter by TET enzyme activity increases transcription of the gene.
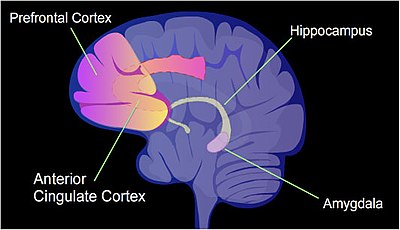
Transcriptional regulation in learning and memory
The identified areas of the human brain are involved in memory formation.
In a rat, contextual fear conditioning (CFC) is a painful learning experience. Just one episode of CFC can result in a life-long fearful memory. After an episode of CFC, cytosine methylation is altered in the promoter regions of about 9.17% of all genes in the hippocampus neuron DNA of a rat. The hippocampus
is where new memories are initially stored. After CFC about 500 genes
have increased transcription (often due to demethylation of CpG sites in
a promoter region) and about 1,000 genes have decreased transcription
(often due to newly formed 5-methylcytosine at CpG sites in a promoter
region). The pattern of induced and repressed genes within neurons
appears to provide a molecular basis for forming the first transient
memory of this training event in the hippocampus of the rat brain.
In particular, the brain-derived neurotrophic factor gene (BDNF) is known as a "learning gene." After CFC there was upregulation of BDNF
gene expression, related to decreased CpG methylation of certain
internal promoters of the gene, and this was correlated with learning.
Transcriptional regulation in cancer
The majority of gene promoters contain a CpG island with numerous CpG sites. When many of a gene's promoter CpG sites are methylated the gene becomes silenced. Colorectal cancers typically have 3 to 6 driver mutations and 33 to 66 hitchhiker or passenger mutations.
However, transcriptional silencing may be of more importance than
mutation in causing progression to cancer. For example, in colorectal
cancers about 600 to 800 genes are transcriptionally silenced by CpG
island methylation (see regulation of transcription in cancer). Transcriptional repression in cancer can also occur by other epigenetic mechanisms, such as altered expression of microRNAs. In breast cancer, transcriptional repression of BRCA1 may occur more frequently by over-expressed microRNA-182 than by hypermethylation of the BRCA1 promoter (see Low expression of BRCA1 in breast and ovarian cancers).
Post-transcriptional regulation
In eukaryotes, where export of RNA is required before translation is
possible, nuclear export is thought to provide additional control over
gene expression. All transport in and out of the nucleus is via the nuclear pore and transport is controlled by a wide range of importin and exportin proteins.
Expression of a gene coding for a protein is only possible if the
messenger RNA carrying the code survives long enough to be translated.
In a typical cell, an RNA molecule is only stable if specifically
protected from degradation. RNA degradation has particular importance in
regulation of expression in eukaryotic cells where mRNA has to travel
significant distances before being translated. In eukaryotes, RNA is
stabilised by certain post-transcriptional modifications, particularly
the 5′ cap and poly-adenylated tail.
Intentional degradation of mRNA is used not just as a defence
mechanism from foreign RNA (normally from viruses) but also as a route
of mRNA destabilisation. If an mRNA molecule has a complementary sequence to a small interfering RNA then it is targeted for destruction via the RNA interference pathway.
Three prime untranslated regions and microRNAs
Three prime untranslated regions (3′UTRs) of messenger RNAs
(mRNAs) often contain regulatory sequences that post-transcriptionally
influence gene expression. Such 3′-UTRs often contain both binding sites
for microRNAs
(miRNAs) as well as for regulatory proteins. By binding to specific
sites within the 3′-UTR, miRNAs can decrease gene expression of various
mRNAs by either inhibiting translation or directly causing degradation
of the transcript. The 3′-UTR also may have silencer regions that bind
repressor proteins that inhibit the expression of a mRNA.
The 3′-UTR often contains microRNA response elements (MREs).
MREs are sequences to which miRNAs bind. These are prevalent motifs
within 3′-UTRs. Among all regulatory motifs within the 3′-UTRs (e.g.
including silencer regions), MREs make up about half of the motifs.
As of 2014, the miRBase web site, an archive of miRNA sequences
and annotations, listed 28,645 entries in 233 biologic species. Of
these, 1,881 miRNAs were in annotated human miRNA loci. miRNAs were
predicted to have an average of about four hundred target mRNAs (affecting expression of several hundred genes). Friedman et al.
estimate that >45,000 miRNA target sites within human mRNA 3′UTRs
are conserved above background levels, and >60% of human
protein-coding genes have been under selective pressure to maintain
pairing to miRNAs.
Direct experiments show that a single miRNA can reduce the stability of hundreds of unique mRNAs.
Other experiments show that a single miRNA may repress the production
of hundreds of proteins, but that this repression often is relatively
mild (less than 2-fold).
The effects of miRNA dysregulation of gene expression seem to be important in cancer. For instance, in gastrointestinal cancers, nine miRNAs have been identified as epigenetically altered and effective in down regulating DNA repair enzymes.
The effects of miRNA dysregulation of gene expression also seem
to be important in neuropsychiatric disorders, such as schizophrenia,
bipolar disorder, major depression, Parkinson's disease, Alzheimer's
disease and autism spectrum disorders.
Translational regulation
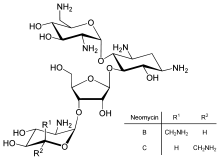
Neomycin
is an example of a small molecule that reduces expression of all
protein genes inevitably leading to cell death; it thus acts as an
antibiotic.
Direct regulation of translation is less prevalent than control of
transcription or mRNA stability but is occasionally used. Inhibition of
protein translation is a major target for toxins and antibiotics, so they can kill a cell by overriding its normal gene expression control. Protein synthesis inhibitors include the antibiotic neomycin and the toxin ricin.
Post-translational modifications
Post-translational modifications (PTMs) are covalent
modifications to proteins. Like RNA splicing, they help to
significantly diversify the proteome. These modifications are usually
catalyzed by enzymes. Additionally, processes like covalent additions to
amino acid side chain residues can often be reversed by other enzymes.
However, some, like the proteolytic cleavage of the protein backbone, are irreversible.
PTMs play many important roles in the cell. For example, phosphorylation is primarily involved in activating and deactivating proteins and in signaling pathways.
PTMs are involved in transcriptional regulation: an important function
of acetylation and methylation is histone tail modification, which
alters how accessible DNA is for transcription. They can also be seen in the immune system, where glycosylation plays a key role. One type of PTM can initiate another type of PTM, as can be seen in how ubiquitination tags proteins for degradation through proteolysis.
Proteolysis, other than being involved in breaking down proteins, is
also important in activating and deactivating them, and in regulating
biological processes such as DNA transcription and cell death.
Measurement
Measuring gene expression is an important part of many life sciences,
as the ability to quantify the level at which a particular gene is
expressed within a cell, tissue or organism can provide a lot of
valuable information. For example, measuring gene expression can:
Similarly, the analysis of the location of protein expression is a
powerful tool, and this can be done on an organismal or cellular scale.
Investigation of localization is particularly important for the study of
development
in multicellular organisms and as an indicator of protein function in
single cells. Ideally, measurement of expression is done by detecting
the final gene product (for many genes, this is the protein); however,
it is often easier to detect one of the precursors, typically mRNA and to infer gene-expression levels from these measurements.
mRNA quantification
Levels of mRNA can be quantitatively measured by northern blotting, which provides size and sequence information about the mRNA molecules. A sample of RNA is separated on an agarose gel
and hybridized to a radioactively labeled RNA probe that is
complementary to the target sequence. The radiolabeled RNA is then
detected by an autoradiograph.
Because the use of radioactive reagents makes the procedure
time-consuming and potentially dangerous, alternative labeling and
detection methods, such as digoxigenin and biotin chemistries, have been
developed. Perceived disadvantages of Northern blotting are that large
quantities of RNA are required and that quantification may not be
completely accurate, as it involves measuring band strength in an image
of a gel. On the other hand, the additional mRNA size information from
the Northern blot allows the discrimination of alternately spliced
transcripts.
Another approach for measuring mRNA abundance is RT-qPCR. In this technique, reverse transcription is followed by quantitative PCR. Reverse transcription first generates a DNA template from the mRNA; this single-stranded template is called cDNA. The cDNA template is then amplified in the quantitative step, during which the fluorescence emitted by labeled hybridization probes or intercalating dyes changes as the DNA amplification
process progresses. With a carefully constructed standard curve, qPCR
can produce an absolute measurement of the number of copies of original
mRNA, typically in units of copies per nanolitre of homogenized tissue
or copies per cell. qPCR is very sensitive (detection of a single mRNA
molecule is theoretically possible), but can be expensive depending on
the type of reporter used; fluorescently labeled oligonucleotide probes
are more expensive than non-specific intercalating fluorescent dyes.
For expression profiling, or high-throughput analysis of many genes within a sample, quantitative PCR may be performed for hundreds of genes simultaneously in the case of low-density arrays. A second approach is the hybridization microarray.
A single array or "chip" may contain probes to determine transcript
levels for every known gene in the genome of one or more organisms.
Alternatively, "tag based" technologies like Serial analysis of gene expression (SAGE) and RNA-Seq, which can provide a relative measure of the cellular concentration
of different mRNAs, can be used. An advantage of tag-based methods is
the "open architecture", allowing for the exact measurement of any
transcript, with a known or unknown sequence. Next-generation sequencing
(NGS) such as RNA-Seq
is another approach, producing vast quantities of sequence data that
can be matched to a reference genome. Although NGS is comparatively
time-consuming, expensive, and resource-intensive, it can identify single-nucleotide polymorphisms,
splice-variants, and novel genes, and can also be used to profile
expression in organisms for which little or no sequence information is
available.
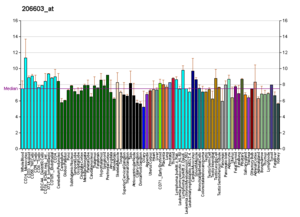
RNA profiles in Wikipedia
The RNA expression profile of the GLUT4 Transporter (one of the main glucose transporters found in the human body)
Profiles like these are found for almost all proteins listed in Wikipedia. They are generated by organizations such as the Genomics Institute of the Novartis Research Foundation and the European Bioinformatics Institute.
Additional information can be found by searching their databases (for
an example of the GLUT4 transporter pictured here, see citation).
These profiles indicate the level of DNA expression (and hence RNA
produced) of a certain protein in a certain tissue, and are color-coded
accordingly in the images located in the Protein Box on the right side
of each Wikipedia page.
Protein quantification
For
genes encoding proteins, the expression level can be directly assessed
by a number of methods with some clear analogies to the techniques for
mRNA quantification.
One of the most commonly used methods is to perform a Western blot against the protein of interest. This gives information on the size of the protein in addition to its identity. A sample (often cellular lysate) is separated on a polyacrylamide gel, transferred to a membrane and then probed with an antibody to the protein of interest. The antibody can either be conjugated to a fluorophore or to horseradish peroxidase
for imaging and/or quantification. The gel-based nature of this assay
makes quantification less accurate, but it has the advantage of being
able to identify later modifications to the protein, for example
proteolysis or ubiquitination, from changes in size.
mRNA-protein correlation
Quantification
of protein and mRNA permits a correlation of the two levels. The
question of how well protein levels correlate with their corresponding
transcript levels is highly debated and depends on multiple factors.
Regulation on each step of gene expression can impact the correlation,
as shown for regulation of translation or protein stability. Post-translational factors, such as protein transport in highly polar cells, can influence the measured mRNA-protein correlation as well.
Localisation
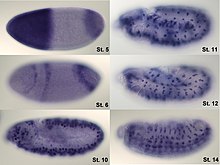
In situ-hybridization of
Drosophila embryos at different developmental stages for the mRNA responsible for the expression of
hunchback. High intensity of blue color marks places with high hunchback mRNA quantity.
Analysis of expression is not limited to quantification; localisation
can also be determined. mRNA can be detected with a suitably labelled
complementary mRNA strand and protein can be detected via labelled
antibodies. The probed sample is then observed by microscopy to identify
where the mRNA or protein is.
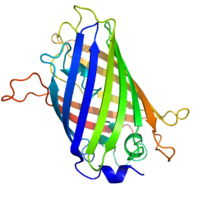
The three-dimensional structure of
green fluorescent protein.
The residues in the centre of the "barrel" are responsible for
production of green light after exposing to higher energetic blue light.
From
PDB: 1EMA.
By replacing the gene with a new version fused to a green fluorescent protein (or similar) marker, expression may be directly quantified in live cells. This is done by imaging using a fluorescence microscope.
It is very difficult to clone a GFP-fused protein into its native
location in the genome without affecting expression levels so this
method often cannot be used to measure endogenous gene expression. It
is, however, widely used to measure the expression of a gene
artificially introduced into the cell, for example via an expression vector.
It is important to note that by fusing a target protein to a
fluorescent reporter the protein's behavior, including its cellular
localization and expression level, can be significantly changed.
The enzyme-linked immunosorbent assay works by using antibodies immobilised on a microtiter plate
to capture proteins of interest from samples added to the well. Using a
detection antibody conjugated to an enzyme or fluorophore the quantity
of bound protein can be accurately measured by fluorometric or colourimetric
detection. The detection process is very similar to that of a Western
blot, but by avoiding the gel steps more accurate quantification can be
achieved.
Expression system
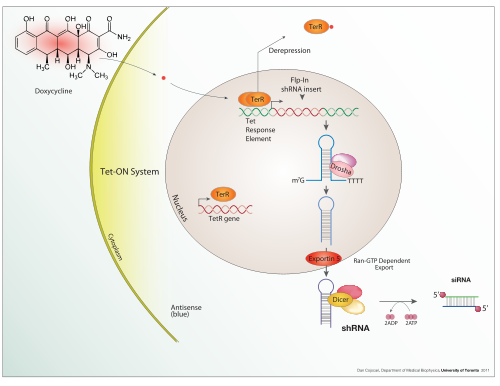
Tet-ON inducible shRNA system
An expression system is a system specifically designed for the
production of a gene product of choice. This is normally a protein
although may also be RNA, such as tRNA or a ribozyme. An expression system consists of a gene, normally encoded by DNA, and the molecular machinery required to transcribe the DNA into mRNA and translate the mRNA into protein
using the reagents provided. In the broadest sense this includes every
living cell but the term is more normally used to refer to expression as
a laboratory tool. An expression system is therefore often artificial
in some manner. Expression systems are, however, a fundamentally natural
process. Viruses are an excellent example where they replicate by using
the host cell as an expression system for the viral proteins and
genome.
Inducible expression
Doxycycline is also used in "Tet-on" and "Tet-off" tetracycline controlled transcriptional activation to regulate transgene expression in organisms and cell cultures.
In nature
In
addition to these biological tools, certain naturally observed
configurations of DNA (genes, promoters, enhancers, repressors) and the
associated machinery itself are referred to as an expression system.
This term is normally used in the case where a gene or set of genes is
switched on under well defined conditions, for example, the simple
repressor switch expression system in Lambda phage and the lac operator
system in bacteria. Several natural expression systems are directly
used or modified and used for artificial expression systems such as the Tet-on and Tet-off expression system.
Gene networks
Genes have sometimes been regarded as nodes in a network, with inputs being proteins such as transcription factors,
and outputs being the level of gene expression. The node itself
performs a function, and the operation of these functions have been
interpreted as performing a kind of information processing within cells and determines cellular behavior.
Gene networks can also be constructed without formulating an
explicit causal model. This is often the case when assembling networks
from large expression data sets. Covariation and correlation of expression is computed across a large sample of cases and measurements (often transcriptome or proteome
data). The source of variation can be either experimental or natural
(observational). There are several ways to construct gene expression
networks, but one common approach is to compute a matrix of all
pair-wise correlations of expression across conditions, time points, or
individuals and convert the matrix (after thresholding at some cut-off
value) into a graphical representation in which nodes represent genes,
transcripts, or proteins and edges connecting these nodes represent the
strength of association.
Techniques and tools
The
following experimental techniques are used to measure gene expression
and are listed in roughly chronological order, starting with the older,
more established technologies. They are divided into two groups based on
their degree of multiplexity.
- Low-to-mid-plex techniques:
- Higher-plex techniques:
Gene expression databases