| Blood-brain barrier | |
|---|---|
 Solute permeability at the BBB vs. choroid plexus | |
| Details | |
| System | Neuroimmune system |
| Identifiers | |
| Acronym(s) | BBB |
| MeSH | D001812 |
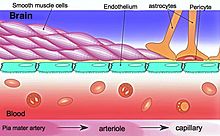
The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system where neurons reside. The blood-brain barrier is formed by endothelial cells of the capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some molecules by passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose, water and amino acids that are crucial to neural function.
The blood-brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and large or hydrophilic molecules into the cerebrospinal fluid, while allowing the diffusion of hydrophobic molecules (O2, CO2, hormones) and small polar molecules. Cells of the barrier actively transport metabolic products such as glucose across the barrier using specific transport proteins. The barrier also restricts the passage of peripheral immune factors, like signaling molecules, antibodies, and immune cells, into the CNS, thus insulating the brain from damage due to peripheral immune events.
Specialized brain structures participating in sensory and secretory integration within brain neural circuits—the circumventricular organs and choroid plexus—have highly permeable capillaries.
Structure
The blood–brain barrier results from the selectivity of the tight junctions between the endothelial cells of brain capillaries, restricting the passage of solutes. At the interface between blood and the brain, endothelial cells are adjoined continuously by these tight junctions, which are composed of smaller subunits of transmembrane proteins, such as occludin, claudins, junctional adhesion molecule. Each of these transmembrane proteins is anchored into the endothelial cells by another protein complex that includes tight junction protein 1 and associated proteins.
The blood-brain barrier is composed of endothelial cells restricting passage of substances from the blood more selectively than endothelial cells of capillaries elsewhere in the body. Astrocyte cell projections called astrocytic feet (also known as "glia limitans") surround the endothelial cells of the BBB, providing biochemical support to those cells. The BBB is distinct from the quite similar blood-cerebrospinal fluid barrier, which is a function of the choroidal cells of the choroid plexus, and from the blood-retinal barrier, which can be considered a part of the whole realm of such barriers.
Several areas of the human brain are not on the brain side of the BBB. Some examples of this include the circumventricular organs, the roof of the third and fourth ventricles, capillaries in the pineal gland on the roof of the diencephalon and the pineal gland. The pineal gland secretes the hormone melatonin "directly into the systemic circulation", thus melatonin is not affected by the blood-brain barrier.
Development
The blood-brain barrier appears to be functional by the time of birth. P-glycoprotein, a transporter, exists already in the embryonal endothelium.
Measurement of brain uptake of various blood-borne solutes showed that newborn endothelial cells were functionally similar to those in adults, indicating that a selective BBB is operative at birth.
Function
The blood-brain barrier acts effectively to protect the brain from circulating pathogens. Accordingly, blood-borne infections of the brain are rare. Infections of the brain that do occur are often difficult to treat. Antibodies are too large to cross the blood-brain barrier, and only certain antibiotics are able to pass. In some cases, a drug has to be administered directly into the cerebrospinal fluid where it can enter the brain by crossing the blood-cerebrospinal fluid barrier.
The blood-brain barrier may become leaky in select neurological diseases, such as amyotrophic lateral sclerosis, epilepsy, brain trauma and edema, and in systemic diseases, such as liver failure. The blood-brain barrier becomes more permeable during inflammation, potentially allowing antibiotics and phagocytes to move across the BBB.
Circumventricular organs
Circumventricular organs (CVOs) are individual structures located adjacent to the fourth ventricle or third ventricle in the brain, and are characterized by dense capillary beds with permeable endothelial cells unlike those of the blood-brain barrier. Included among CVOs having highly permeable capillaries are the area postrema, subfornical organ, vascular organ of the lamina terminalis, median eminence, pineal gland, and three lobes of the pituitary gland.
Permeable capillaries of the sensory CVOs (area postrema, subfornical organ, vascular organ of the lamina terminalis) enable rapid detection of circulating signals in systemic blood, while those of the secretory CVOs (median eminence, pineal gland, pituitary lobes) facilitate transport of brain-derived signals into the circulating blood. Consequently, the CVO permeable capillaries are the point of bidirectional blood-brain communication for neuroendocrine function.
Specialized permeable zones
The border zones between brain tissue "behind" the blood-brain barrier and zones "open" to blood signals in certain CVOs contain specialized hybrid capillaries that are leakier than typical brain capillaries, but not as permeable as CVO capillaries. Such zones exist at the border of the area postrema—nucleus tractus solitarii (NTS), and median eminence—hypothalamic arcuate nucleus. These zones appear to function as rapid transit regions for brain structures involved in diverse neural circuits—like the NTS and arcuate nucleus—to receive blood signals which are then transmitted into neural output. The permeable capillary zone shared between the median eminence and hypothalamic arcuate nucleus is augmented by wide pericapillary spaces, facilitating bidirectional flow of solutes between the two structures, and indicating that the median eminence is not only a secretory organ, but may also be a sensory organ.
Therapeutic research
As a drug target
The blood-brain barrier is formed by the brain capillary endothelium and excludes from the brain 100% of large-molecule neurotherapeutics and more than 98% of all small-molecule drugs. Overcoming the difficulty of delivering therapeutic agents to specific regions of the brain presents a major challenge to treatment of most brain disorders. In its neuroprotective role, the blood-brain barrier functions to hinder the delivery of many potentially important diagnostic and therapeutic agents to the brain. Therapeutic molecules and antibodies that might otherwise be effective in diagnosis and therapy do not cross the BBB in adequate amounts to be clinically effective.
Mechanisms for drug targeting in the brain involve going either "through" or "behind" the BBB. Modalities for drug delivery to the brain in unit doses through the BBB entail its disruption by osmotic means, or biochemically by the use of vasoactive substances, such as bradykinin, or even by localized exposure to high-intensity focused ultrasound (HIFU).
Other methods used to get through the BBB may entail the use of endogenous transport systems, including carrier-mediated transporters, such as glucose and amino acid carriers, receptor-mediated transcytosis for insulin or transferrin, and the blocking of active efflux transporters such as p-glycoprotein. Some studies have shown that vectors targeting BBB transporters, such as the transferrin receptor, have been found to remain entrapped in brain endothelial cells of capillaries, instead of being ferried across the BBB into the targeted area.
Nanoparticles
Nanotechnology is under preliminary research for its potential to facilitate the transfer of drugs across the BBB. Capillary endothelial cells and associated pericytes may be abnormal in tumors and the blood-brain barrier may not always be intact in brain tumors. Other factors, such as astrocytes, may contribute to the resistance of brain tumors to therapy using nanoparticles. Fat soluble molecules less than 400 Daltons in weight can freely diffuse past the BBB through lipid mediated passive diffusion.
History
Paul Ehrlich was a bacteriologist studying staining, a procedure that is used in many microscopy studies to make fine biological structures visible using chemical dyes. As Ehrlich injected some of these dyes (notably the aniline dyes that were then widely used), the dye stained all of the organs of some kinds of animals except for their brains. At that time, Ehrlich attributed this lack of staining to the brain simply not picking up as much of the dye.
However, in a later experiment in 1913, Edwin Goldmann (one of Ehrlich's students) injected the dye directly into the cerebrospinal fluids of animal brains. He found then the brains did become dyed, but the rest of the body did not, demonstrating the existence of a compartmentalization between the two. At that time, it was thought that the blood vessels themselves were responsible for the barrier, since no obvious membrane could be found. The concept of the blood–brain barrier (then termed hematoencephalic barrier) was proposed by a Berlin physician, Lewandowsky, in 1900.