From Wikipedia, the free encyclopedia
Carbon capture and storage (CCS) (or carbon capture and sequestration) is the process of capturing waste carbon dioxide (CO
2) from large point sources, such as fossil fuel power plants, transporting it to a storage site, and depositing it where it will not enter the atmosphere, normally an underground geological formation. The aim is to prevent the release of large quantities of CO
2 into the atmosphere (from fossil fuel use in power generation and other industries). It is a potential means of mitigating the contribution of fossil fuel emissions to global warming[1] and ocean acidification.[2] Although CO
2 has been injected into geological formations for several decades for various purposes, including enhanced oil recovery, the long term storage of CO
2 is a relatively new concept. The first commercial example was Weyburn in 2000.[3] 'CCS' can also be used to describe the scrubbing of CO
2 from ambient air as a climate engineering technique.
An integrated pilot-scale CCS power plant was to begin operating in September 2008 in the eastern German power plant Schwarze Pumpe run by utility Vattenfall, in the hope of answering questions about technological feasibility and economic efficiency. CCS applied to a modern conventional power plant could reduce CO
2 emissions to the atmosphere by approximately 80–90% compared to a plant without CCS.[4] The IPCC estimates that the economic potential of CCS could be between 10% and 55% of the total carbon mitigation effort until year 2100.[4]
Capturing and compressing CO
2 may increase the fuel needs of a coal-fired CCS plant by 25–40%.[4] These and other system costs are estimated to increase the cost of the energy produced by 21–91% for purpose built plants.[4] Applying the technology to existing plants would be more expensive especially if they are far from a sequestration site. Recent industry reports suggest that with successful research, development and deployment (RD&D), sequestered coal-based electricity generation in 2025 may cost less than unsequestered coal-based electricity generation today.[5]
Storage of the CO
2 is envisaged either in deep geological formations, or in the form of mineral carbonates. Deep ocean storage is no longer considered feasible because it greatly increases the problem of ocean acidification.[6] Geological formations are currently considered the most promising sequestration sites. The National Energy Technology Laboratory (NETL) reported that North America has enough storage capacity for more than 900 years worth of carbon dioxide at current production rates.[7] A general problem is that long term predictions about submarine or underground storage security are very difficult and uncertain, and there is still the risk that CO
2 might leak into the atmosphere.[8]
Capture
Capturing CO2 is probably most effective at point sources, such as large fossil fuel or biomass energy facilities, industries with major CO
2 emissions, natural gas processing, synthetic fuel plants and fossil fuel-based hydrogen production plants. Extraction (recovery) from air is possible, but not very practical. The CO
2 concentration drops rapidly moving away from the point source. The lower concentration increases the amount of mass flow that must be processed (per tonne of carbon dioxide extracted).[9]
Concentrated CO
2 from the combustion of coal in oxygen is relatively pure, and could be directly processed. Impurities in CO
2 streams could have a significant effect on their phase behaviour and could pose a significant threat of increased corrosion of pipeline and well materials.[10] In instances where CO
2 impurities exist and especially with air capture, a scrubbing process would be needed.[11]
Organisms that produce ethanol by fermentation generate cool, essentially pure CO
2 that can be pumped underground.[12] Fermentation produces slightly less CO
2 than ethanol by weight.
Broadly, three different types of technologies for scrubbing exist: post-combustion, pre-combustion, and oxyfuel combustion:
- In post combustion capture, the CO
2 is removed after combustion of the fossil fuel — this is the scheme that would be applied to fossil-fuel burning power plants. Here, carbon dioxide is captured from flue gases at power stations or other large point sources. The technology is well understood and is currently used in other industrial applications, although not at the same scale as might be required in a commercial scale power station.
- The technology for pre-combustion is widely applied in fertilizer, chemical, gaseous fuel (H2, CH4), and power production.[13] In these cases, the fossil fuel is partially oxidized, for instance in a gasifier. The resulting syngas (CO and H2) is shifted into CO
2 and H2. The resulting CO
2 can be captured from a relatively pure exhaust stream. The H2 can now be used as fuel; the carbon dioxide is removed before combustion takes place. There are several advantages and disadvantages when compared to conventional post combustion carbon dioxide capture.[14][15] The CO
2 is removed after combustion of fossil fuels, but before the flue gas is expanded to atmospheric pressure. This scheme is applied to new fossil fuel burning power plants, or to existing plants where re-powering is an option. The capture before expansion, i.e. from pressurized gas, is standard in almost all industrial CO
2 capture processes, at the same scale as will be required for utility power plants.[16][17]
- In oxy-fuel combustion[18] the fuel is burned in oxygen instead of air. To limit the resulting flame temperatures to levels common during conventional combustion, cooled flue gas is recirculated and injected into the combustion chamber. The flue gas consists of mainly carbon dioxide and water vapour, the latter of which is condensed through cooling. The result is an almost pure carbon dioxide stream that can be transported to the sequestration site and stored. Power plant processes based on oxyfuel combustion are sometimes referred to as "zero emission" cycles, because the CO
2 stored is not a fraction removed from the flue gas stream (as in the cases of pre- and post-combustion capture) but the flue gas stream itself. A certain fraction of the CO
2 generated during combustion will inevitably end up in the condensed water. To warrant the label "zero emission" the water would thus have to be treated or disposed of appropriately. The technique is promising, but the initial air separation step demands a lot of energy.
2.[19]
A few engineering proposals have been made for the more difficult task of capturing CO
2 directly from the air, but work in this area is still in its infancy. Capture costs are estimated to be higher than from point sources, but may be feasible for dealing with emissions from diffuse sources such as automobiles and aircraft.[20] The theoretically required energy for air capture is only slightly more than for capture from point sources. The additional costs come from the devices that use the natural air flow. Global Research Technologies demonstrated a pre-prototype of air capture technology in 2007.[21]
Removing CO
2 from the atmosphere is a form of climate engineering by greenhouse gas remediation. Some media reports[citation needed] have suggested that techniques of this type, if coupled with effective carbon sequestration technologies, may offer a comprehensive solution to global warming.
It is more usual to see such techniques proposed for air capture, than for flue gas treatment. Carbon dioxide capture and storage is more commonly proposed on plants burning coal in oxygen extracted from the air, which means the CO
2 is highly concentrated and no scrubbing process is necessary. According to the Wallula Energy Resource Center in Washington state, by gasifying coal, it is possible to capture approximately 65% of carbon dioxide embedded in it and sequester it in a solid form.[22]
Transport
After capture, the CO2 would have to be transported to suitable storage sites. This is done by pipeline, which is generally the cheapest form of transport. In 2008, there were approximately 5,800 km of CO
2 pipelines in the United States, used to transport CO
2 to oil production fields where it is then injected into older fields to extract oil. The injection of CO
2 to produce oil is generally called Enhanced Oil Recovery or EOR.[citation needed] In addition, there are several pilot programs in various stages to test the long-term storage of CO
2 in non-oil producing geologic formations.
According to the Congressional Research Service, "There are important unanswered questions about pipeline network requirements, economic regulation, utility cost recovery, regulatory classification of CO
2 itself, and pipeline safety. Furthermore, because CO
2 pipelines for enhanced oil recovery are already in use today, policy decisions affecting CO
2 pipelines take on an urgency that is unrecognized by many. Federal classification of CO
2 as both a commodity (by the Bureau of Land Management) and as a pollutant (by the Environmental Protection Agency) could potentially create an immediate conflict which may need to be addressed not only for the sake of future CCS implementation, but also to ensure consistency of future CCS with CO
2 pipeline operations today."[23][24]
Ships could also be utilized for transport where pipelines are not feasible. These methods are currently used for transporting CO
2 for other applications.
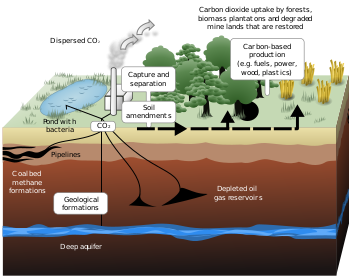
Sequestration
Various forms have been conceived for permanent storage of CO2. These forms include gaseous storage in various deep geological formations (including saline formations and exhausted gas fields), and solid storage by reaction of CO2 with metal oxides to produce stable carbonates.Geological storage
Also known as geo-sequestration, this method involves injecting carbon dioxide, generally in supercritical form, directly into underground geological formations. Oil fields, gas fields, saline formations, unmineable coal seams, and saline-filled basalt formations have been suggested as storage sites. Various physical (e.g., highly impermeable caprock) and geochemical trapping mechanisms would prevent the CO2 from escaping to the surface.[25]
CO
2 is sometimes injected into declining oil fields to increase oil recovery. Approximately 30 to 50 million metric tonnes of CO
2 are injected annually in the United States into declining oil fields.[26] This option is attractive because the geology of hydrocarbon reservoirs is generally well understood and storage costs may be partly offset by the sale of additional oil that is recovered.[27] Disadvantages of old oil fields are their geographic distribution and their limited capacity, as well as the fact that subsequent burning of the additional oil recovered will offset much or all of the reduction in CO
2 emissions.[28]
Unmineable coal seams can be used to store CO
2 because the CO
2 molecules attach to the surface of coal. The technical feasibility, however, depends on the permeability of the coal bed. In the process of absorption the coal releases previously absorbed methane, and the methane can be recovered (enhanced coal bed methane recovery). The sale of the methane can be used to offset a portion of the cost of the CO
2 storage. Burning the resultant methane, however, would negate some of the benefit of sequestering the original CO
2.
Saline formations contain highly mineralized brines, and have so far been considered of no benefit to humans. Saline aquifers have been used for storage of chemical waste in a few cases. The main advantage of saline aquifers is their large potential storage volume and their common occurrence. The major disadvantage of saline aquifers is that relatively little is known about them, especially compared to oil fields. To keep the cost of storage acceptable, the geophysical exploration may be limited, resulting in larger uncertainty about the aquifer structure. Unlike storage in oil fields or coal beds, no side product will offset the storage cost. Leakage of CO
2 back into the atmosphere may be a problem in saline aquifer storage. Current research shows, however, that trapping mechanisms such as structural trapping, residual trapping, solubility trapping and mineral trapping could immobilize the CO
2 underground and reduce the risk of leakage.[25]
Enhanced oil recovery
Enhanced oil recovery (EOR) is a generic term for techniques used to increase the amount of crude oil that can be extracted from an oil field. In Carbon Capture & Sequestration Enhanced Oil Recovery (CCS EOR), carbon dioxide is injected into an oil field to recover oil that is often never recovered using more traditional methods.Crude oil development and production in U.S. oil reservoirs can include up to three distinct phases: primary, secondary, and tertiary (or enhanced) recovery.[29] During primary recovery only about 10 percent of a reservoir's original oil in place is typically produced. Secondary recovery techniques extend a field's productive life generally by injecting water or gas to displace oil and drive it to a production wellbore, resulting in the recovery of 20 to 40 percent of the original oil in place. However, with much of the easy-to-produce oil already recovered from U.S. oil fields, producers have attempted several tertiary, or enhanced oil recovery (EOR), techniques that offer prospects for ultimately producing 30 to 60 percent, or more, of the reservoir's original oil in place.[30]
An example of a project that will use CCS EOR is the Kemper Project in Mississippi. Due to the Kemper Project's close proximity to oil fields, the carbon dioxide byproduct from producing electricity will be transported to the neighboring oil fields for EOR.[31]
Ocean storage
In the past, it was suggested that CO2 could be stored in the oceans, but this would only exacerbate ocean acidification and has been made illegal under specific regulations. Ocean storage is no longer considered feasible.[6]
Mineral storage
In this process, CO2 is exothermically reacted with available metal oxides, which in turn produces stable carbonates. This process occurs naturally over many years and is responsible for a great amount of surface limestone. The idea of using Olivine has been promoted by the geochemist Prof. Schuiling.[32] The reaction rate can be made faster, for example, with a catalyst[33] or by reacting at higher temperatures and/or pressures, or by pre-treatment of the minerals, although this method can require additional energy. The IPCC estimates that a power plant equipped with CCS using mineral storage will need 60–180% more energy than a power plant without CCS.[4]
The economics of mineral carbonation at scale are now being tested in a world-first pilot plant project based in Newcastle, Australia. New techniques for mineral activation and reaction have been developed the GreenMag Group and the University of Newcastle and funded by the New South Wales and Australian Governments to be operational by 2013.[34]
In 2009 it was reported that scientists had mapped 6,000 square miles (16,000 km2) of rock formations in the U.S. that could be used to store 500 years' worth of U.S. carbon dioxide emissions.[35] A study on mineral sequestration in the US states:
Carbon sequestration by reacting naturally occurring Mg and Ca containing minerals with COThe following table lists principal metal oxides of Earth's Crust. Theoretically, up to 22% of this mineral mass is able to form carbonates.
2 to form carbonates has many unique advantages. Most notabl[e] is the fact that carbonates have a lower energy state than CO
2, which is why mineral carbonation is thermodynamically favorable and occurs naturally (e.g., the weathering of rock over geologic time periods). Secondly, the raw materials such as magnesium based minerals are abundant. Finally, the produced carbonates are unarguably stable and thus re-release of CO
2 into the atmosphere is not an issue. However, conventional carbonation pathways are slow under ambient temperatures and pressures. The significant challenge being addressed by this effort is to identify an industrially and environmentally viable carbonation route that will allow mineral sequestration to be implemented with acceptable economics.[36]
| Earthen Oxide | Percent of Crust | Carbonate | Enthalpy change (kJ/mol) |
|---|---|---|---|
| SiO2 | 59.71 | ||
| Al2O3 | 15.41 | ||
| CaO | 4.90 | CaCO3 | -179 |
| MgO | 4.36 | MgCO3 | -118 |
| Na2O | 3.55 | Na2CO3 | -322 |
| FeO | 3.52 | FeCO3 | -85 |
| K2O | 2.80 | K2CO3 | -393.5 |
| Fe2O3 | 2.63 | FeCO3 | 112 |
| 21.76 | All Carbonates |
Ultramafic mine tailings are a readily available source of fine-grained metal oxides that can act as artificial carbon sinks to reduce net greenhouse gas emissions in the mining industry.[37] Accelerating passive CO2 sequestration via mineral carbonation may be achieved through microbial processes that enhance mineral dissolution and carbonate precipitation.[38][39][40]
Energy requirements
The energy requirements of sequestration processes may be significant. In one paper, sequestration consumed 25 percent of the plant's rated 600 megawatt output capacity.[41]- After adding CO
2 capture and compression, the capacity of the coal-fired power plant is reduced to 457 MW.
Leakage
A major concern with CCS is whether leakage of stored CO
2 will compromise CCS as a climate change mitigation option. For well-selected, designed and managed geological storage sites, IPCC estimates that risks are comparable to those associated with current hydrocarbon activity.[42] Although some question this assumption as arbitrary citing a lack of experience in such long term storage.[43][44] CO
2 could be trapped for millions of years, and although some leakage occurs upwards through the soil, well selected storage sites are likely to retain over 99% of the injected CO
2 over 1000 years.[45] Leakage through the injection pipe is a greater risk.[46]
Although the injection pipe is usually protected with non-return valves to prevent release on a power outage, there is still a risk that the pipe itself could tear and leak due to the pressure. The Berkel en Rodenrijs incident in December 2008 was an example, where a modest release of CO
2 from a pipeline under a bridge resulted in the deaths of some ducks sheltering there.[47] In order to measure accidental carbon releases more accurately and decrease the risk of fatalities through this type of leakage, the implementation of CO
2 alert meters around the project perimeter has been proposed. Malfunction of a carbon dioxide industrial fire suppression system in a large warehouse released CO
2 and 14 citizens collapsed on the nearby public road. A release of CO
2 from a salt mine killed a person at distance of 300 meters.[48]
In 1986 a large leakage of naturally sequestered CO
2 rose from Lake Nyos in Cameroon and asphyxiated 1,700 people. While the carbon had been sequestered naturally, some point to the event as evidence for the potentially catastrophic effects of sequestering carbon artificially.[49] The Lake Nyos disaster resulted from a volcanic event, which very suddenly released as much as a cubic kilometre of CO
2 gas from a pool of naturally occurring CO
2 under the lake in a deep narrow valley. The location of this pool of CO
2 is not a place where man can inject or store CO
2, and this pool was not known about nor monitored until after the occurrence of the natural disaster.
For ocean storage, the retention of CO
2 would depend on the depth. The IPCC estimates 30–85% of the sequestered carbon dioxide would be retained after 500 years for depths 1000–3000 m. Mineral storage is not regarded as having any risks of leakage. The IPCC recommends that limits be set to the amount of leakage that can take place. This might rule out deep ocean storage as an option.
At the conditions of the deeper oceans, (about 400 bar or 40 MPa, 280 K) water–CO
2(l) mixing is very low (where carbonate formation/acidification is the rate limiting step), but the formation of water-CO
2 hydrates, a kind of solid water cage that surrounds the CO
2, is favorable.
To further investigate the safety of CO
2 sequestration, Norway's Sleipner gas field can be studied, as it is the oldest plant that stores CO
2 on an industrial scale. According to an environmental assessment of the gas field which was conducted after ten years of operation, the author affirmed that geosequestration of CO
2 was the most definite form of permanent geological storage of CO
2:
Available geological information shows absence of major tectonic events after the deposition of the Utsira formation [saline reservoir]. This implies that the geological environment is tectonically stable and a site suitable for carbon dioxide storage. The solubility trapping [is] the most permanent and secure form of geological storage.[50]In March 2009 StatoilHydro issued a study showing the slow spread of CO
2 in the formation after more than 10 years operation.[51]
Phase I of the Weyburn-Midale Carbon Dioxide Project in Weyburn, Saskatchewan, Canada has determined that the likelihood of stored CO
2 release is less than one percent in 5,000 years.[52] A January 2011 report, however, claimed evidence of leakage in land above that project.[53] This report was strongly refuted by the IEAGHG Weyburn-Midale CO
2 Monitoring and Storage Project, which issued an eight page analysis of the study, claiming that it showed no evidence of leakage from the reservoir.[54]
The liability of potential leak(s) is one of the largest barriers to large-scale CCS. To assess and reduce such liability, the leakage of stored gasses, particularly carbon dioxide, into the atmosphere may be detected via atmospheric gas monitoring, and can be quantified directly via the eddy covariance flux measurements,[55][56][57]
Carbon dioxide recycling / Carbon Capture and Utilization (CCU)
Recycling CO2 may offer a response to the global challenge of significantly reducing greenhouse gas emissions from major stationary (industrial) emitters in the near to medium term,[citation needed] but is usually considered a different technological category from CCS.[58] Technologies under development, such as Bio CCS Algal Synthesis,[59] utilises pre-smokestack CO
2 (such as from a coal-fired power station) as a useful feedstock input to the production of oil-rich algae in solar membranes to produce oil for plastics and transport fuel (including aviation fuel), and nutritious stock-feed for farm animal production.[59] The CO
2 and other captured greenhouse gases are injected into the membranes containing waste water and select strains of algae causing, together with sunlight or UV light, an oil rich biomass that doubles in mass every 24 hours[citation needed].
The Bio CCS Algal Synthesis process is based on earth science photosynthesis: the technology is entirely retrofittable and collocated with the emitter, and the capital outlays may offer a return upon investment due to the high value commodities produced (oil for plastics, fuel and feed).
Bio CCS Algal Synthesis test facilities are being trialed at Australia's three largest coal-fired power stations (Tarong, Queensland; Eraring, NSW; Loy Yang, Victoria) using piped pre-emission smokestack CO
2 (and other greenhouse gases) as feedstock to grow oil-rich algal biomass in enclosed membranes for the production of plastics, transport fuel and nutritious animal feed.
Another potentially useful way of dealing with industrial sources of CO
2 is to convert it into hydrocarbons where it can be stored or reused as fuel or to make plastics. There are a number of projects investigating this possibility.[60]
Carbon dioxide scrubbing variants exist based on potassium carbonate which can be used to create liquid fuels, though this process requires a great deal of energy input.[61] Although the creation of fuel from atmospheric CO
2 is not a climate engineering technique, nor does it actually function as greenhouse gas remediation, it nevertheless is potentially useful in the creation of a low carbon economy.
Other uses are the production of stable carbonates from silicates (i.e. Olivine produces Magnesium carbonate. This process is still in the R&D phase.[62]
Single step methods: methanol
A proven process to produce a hydrocarbon is to make methanol. Methanol is rather easily synthesized from CO2 and H2 (See Green Methanol Synthesis). Based on this fact the idea of a methanol economy was born.
Single step methods: hydrocarbons
At the department of Industrial Chemistry and Engineering of Materials at the University of Messina, Italy, there is a project to develop a system which works like a fuel-cell in reverse, whereby a catalyst is used that enables sunlight to split water into hydrogen ions and oxygen gas. The ions cross a membrane where they react with the CO2 to create hydrocarbons.[63]
Two step methods
If CO2 is heated to 2400 °C, it splits into carbon monoxide (CO) and oxygen. The Fischer-Tropsch process can then be used to convert the CO into hydrocarbons. The required temperature can be achieved by using a chamber containing a mirror to focus sunlight on the gas. Rival teams are developing such chambers, at Solarec and at Sandia National Laboratories, both based in New Mexico.[64] According to Sandia these chambers could provide enough fuel to power 100% of domestic vehicles using 5800 km2; unlike biofuels this would not take fertile land away from crops but would be land that is not being used for anything else. James May, the British TV presenter, visited a demonstration plant in a programme in his 'Big Ideas' series.
Example CCS projects
Industrial-scale projects
As at September 2012, the Global CCS Institute identified 75 large-scale integrated projects in its 2012 Global Status of CCS report[65] which is a net increase of one project since its 2011 Global Status of CCS report. 16 of these projects are in operation or in construction capturing around 36 million tonnes of CO2 per annum.[65][66] For more information see Integrated CCS Projects[67] on the Global CCS Institute's website. For information on EU projects see Zero Emissions Platform[68] website. The eight large-scale integrated CCS projects currently in operation are:
1. In Salah CO
2 Injection — Algeria
In Salah is a fully operational onshore gas field with CO2 injection. CO
2 is separated from produced gas and reinjected in the producing hydrocarbon reservoir zones. Since 2004, about 1 Mt/a of CO
2 has been captured during natural gas extraction and injected into the Krechba geologic formation at a depth of 1,800m. The Krechba formation is expected to store 17Mt CO
2 over the life of the project.
2. Sleipner CO
2 Injection — Norway
Sleipner[51] is a fully operational offshore gas field with CO2 injection initiated in 1996. CO2 is separated from produced gas and reinjected in the Utsira saline aquifer (800–1000 m below ocean floor) above the hydrocarbon reservoir zones. This aquifer extends much further north from the Sleipner facility at its southern extreme. The large size of the reservoir accounts for why 600 billion tonnes of CO2 are expected to be stored, long after the Sleipner natural gas project has ended.
3. Snøhvit CO
2 Injection — Norway
Snøhvit is a fully operational offshore gas field with CO2 injection. The LNG plant is located onshore. CO
2 is necessarily separated to produce liquefied natural gas (LNG) and then CO
2 is injected in a saline aquifer below the hydrocarbon reservoir zones offshore at a rate of 700,000 t/a into the Tubåen sandstone formation 2,600 m under the seabed for storage. This formation was closed
April 2011, and injection started in the Stø-formation where produced gas is taken. Produced CO
2 is increasing, therefore separation capacity may limit production before end 2015 when a new formation will be drilled for CO
2-injection only. (Teknisk Ukeblad nr. 30, 2013, tu.no)
4. Great Plains Synfuel Plant and Weyburn-Midale Project — Canada
Weyburn-Midale is a coal gasification operation that produces synthetic natural gas and various petrochemicals from coal. This project captures about 2.8 Mt/a of CO2 from its coal gasification plant located in North Dakota, USA, transported by pipeline 320 km across the Canadian border and injects it into depleting oil fields in Saskatchewan where it is used for enhanced oil recovery (EOR).
5. Shute Creek Gas Processing Facility — USA
Around 7 million tonnes per annum of carbon dioxide are recovered from ExxonMobil's Shute Creek gas processing plant in Wyoming, and transported by pipeline to various oil fields for enhanced oil recovery. This project has been operational since 1986.6. Enid Fertilizer — USA
The Enid Fertilizer plant sends 675,000 tonnes of CO2 to be used for EOR. The pipeline and wells are operated separately by Anadarko Petroleum.
7. Val Verde Natural Gas Plants — USA
CO2 from Mitchell, Gray Ranch, Puckett, and Turrell gas processing plants is transported via the Val Verde and CRC pipelines[where?] for EOR (incl. Sharon Ridge[clarification needed] EOR field).[citation needed]
8. Century Plant — USA
Occidental Petroleum, along with Sandridge Energy, is operating a West Texas hydrocarbon gas processing plant and related pipeline infrastructure that provides CO2 for use in EOR. With a total CO2 capture capacity of 8.5 Mt/a expected in 2012, the Century plant would be the largest single industrial source CO2 capture facility in North America.
Canada
The federal government in the 2008 and 2009 budgets has invested approximately $1.4 billion in Carbon Capture and Storage development.[69]Alberta
Alberta has committed $170 million in 2013/2014 – and a total of $1.3 billion over 15 years – to fund two large-scale CCS projects that will help reduce CO2 emissions from oil sands refining.[70] In 2010 a grant agreement was signed with the Alberta Carbon Trunk Line.[71] The second is the Quest Project.[72]
British Columbia
Spectra Energy's Fort Nelson Project is proposed but still needs to secure funding.[73]Saskatchewan
Led by the province's full-service utility, SaskPower, one of the world's first and largest full production carbon capture facilities is operating at Boundary Dam Power Station. With an initial investment of $1.5 to $1.6 billion,[74] SaskPower will be generating a revenue by selling a portion of the captured CO2 back into the market to be used for enhanced oil recovery. The project started in May 2011 and became operational in October 2014.[75] The post-combustion full flue gas capture process will capture 1 million tonnes of CO
2 a year.[73]
Pilot Projects
The Alberta Saline Aquifer Project (ASAP), Husky Upgrader and Ethanol Plant pilot, Heartland Area Redwater Project (HARP), Wabamun Area Sequestration Project(WASP), and Aquistore.[76]Another Canadian initiative is the Integrated CO
2 Network (ICO
2N), a group of industry participants providing a framework for carbon capture and storage development in Canada.[77] Other Canadian organizations related to CCS include CCS 101, Carbon Management Canada, IPAC CO
2, and the Canadian Clean Power Coalition.[78]
Netherlands
In the Netherlands, a 68 megawatt oxyfuel plant ("Zero Emission Power Plant") was being planned to be operational in 2009.[79] This project was later canceled.ROAD (Rotterdam Capture and Storage Demonstration project) is a joint project by E.ON Benelux and Electrabel Nederland / GDF SUEZ Group. Every year, starting in 2015 ROAD will capture around 1.1 million tonnes of CO
2 at the new power plant on the Maasvlakte. This will be stored in depleted gas reservoirs under the North Sea.[80]
Developed in the Netherlands, an electrocatalysis by a copper complex helps reduce carbon dioxide to oxalic Acid.[81]
Norway
In Norway, the CO2 Technology Centre (TCM) at Mongstad began construction in 2009, and completed in 2012. It includes two capture technology plants (one advanced amine and one chilled ammonia), both capturing fluegas from two sources. This includes a gas-fired power plant and refinery cracker fluegas (similar to coal-fired power plant fluegas).
In addition to this, the Mongstad site was also planned to have a full-scale CCS demonstration plant. The project was delayed to 2014, 2018, and then indefinitely.[82] The project cost rose to USD 985 million.[83] Then in October 2011, Aker Solutions' wrote off its investment in Aker Clean Carbon, declaring the carbon sequestration market to be "dead".[84]
On 1 October 2013 Norway asked Gassnova not to sign any contracts for Carbon capture and storage outside Mongstad.[85]
Poland
In Belchatów, Poland,[86] a lignite-fired energy plant of more than 858 MW is planned to be in operation in 2013.[87][88]United States
In October 2007, the Bureau of Economic Geology at the University of Texas at Austin received a 10-year, $38 million subcontract to conduct the first intensively monitored long-term project in the United States studying the feasibility of injecting a large volume of CO2 for underground storage.[89] The project is a research program of the Southeast Regional Carbon Sequestration Partnership (SECARB), funded by the National Energy Technology Laboratory of the U.S. Department of Energy (DOE).
The SECARB partnership will demonstrate CO
2 injection rate and storage capacity in the Tuscaloosa-Woodbine geologic system that stretches from Texas to Florida. The region has the potential to store more than 200 billion tons[vague] of CO
2 from major point sources in the region, equal to about 33 years of overall U.S. emissions at present rates. Beginning in fall 2007, the project will inject CO
2 at the rate of one million tons[vague] per year, for up to 1.5 years, into brine up to 10,000 feet (3,000 m) below the land surface near the Cranfield oil field, which lays about 15 miles (24 km) east of Natchez, Mississippi. Experimental equipment will measure the ability of the subsurface to accept and retain CO
2.
Currently, the United States government has approved the construction of what is touted as the world's first CCS power plant, FutureGen. On January 29, 2008, however, the Department of Energy announced it was recasting the FutureGen project, and, on June 24, 2008, DoE published a funding opportunity announcement seeking proposals for an IGCC project, with integrated CCS, of at least 250MW.[90] In 2013 and 2013, the FutureGen Alliance proposed a new location in Morgan County, Illinois as an underground storage location for carbon dioxide captured from a power plant retrofitted for oxy-fuel combustion of coal. The liquified carbon dioxide would be transported through about 30 miles of pipeline and injected into four underground injection wells.[91][92]
Examples of carbon sequestration at an existing US coal plant can be found at utility company Luminant's pilot version at its Big Brown Steam Electric Station in Fairfield, Texas. This system is converting carbon from smokestacks into baking soda. Skyonic plans to circumvent storage problems of liquid CO
2 by storing baking soda in mines, landfills, or simply to be sold as industrial or food grade baking soda.[93] Green Fuel Technologies is piloting and implementing algae based carbon capture, circumventing storage issues by then converting algae into fuel or feed.[94]
In November 2008, the DOE awarded a $66.9 million eight-year grant to a research partnership headed by Montana State University to demonstrate that underground geologic formations "can store huge volumes of carbon dioxide economically, safely and permanently".[citation needed] Researchers under the Big Sky Regional Carbon Sequestration Project plan to inject up to one million tonnes of CO
2 into sandstone beneath southwestern Wyoming.[95]
In the United States, four different synthetic fuel projects are moving forward, which have publicly announced plans to incorporate carbon capture and storage:
- American Clean Coal Fuels, in their Illinois Clean Fuels (ICF) project, is developing a 30,000-barrel (4,800 m3) per day biomass and coal to liquids project in Oakland, Illinois, which will market the CO
2 created at the plant for enhanced oil recovery applications. By combining sequestration and biomass feedstocks, the ICF project will achieve dramatic reductions in the life-cycle carbon footprint of the fuels they produce. If sufficient biomass is used, the plant should have the capability to go life-cycle carbon negative, meaning that effectively, for each gallon of their fuel that is used, carbon is pulled out of the air, and put into the ground.[96] - Baard Energy, in their Ohio River Clean Fuels project, is developing a 53,000 bbl/d (8,400 m3/d) coal and biomass to liquids project, which has announced plans to market the plant's CO
2 for enhanced oil recovery.[97] - Rentech is developing a 29,600-barrel (4,710 m3) per day coal and biomass to liquids plant in Natchez, Mississippi, which will market the plant's CO
2 for enhanced oil recovery. The first phase of the project is expected in 2011.[98] - DKRW[who?] is developing a 15,000–20,000-barrel (2,400–3,200 m3) per day coal to liquids plant in Medicine Bow, Wyoming, which will market its plant's CO
2 for enhanced oil recovery. The project is expected to begin operation in 2013.[99]
2 emissions associated with the pulp and paper production industry. The site of the study is the Boise White Paper L.L.C. paper mill located near the township of Wallula in Southeastern Washington State. The plant generates approximately 1.2 MMT of CO
2 annually from a set of three recovery boilers that are mainly fired with black liquor, a recycled byproduct formed during the pulping of wood for paper-making. Fluor Corporation will design a customized version of their Econamine Plus carbon capture technology. The Fluor system also will be designed to remove residual quantities of remnant air pollutants from stack gases as part of the CO
2 capture process. Battelle is leading preparation of an Environmental Information Volume (EIV) for the entire project, including geologic storage of the captured CO
2 in deep flood basalt formations that exist in the greater region. The EIV will describe the necessary site characterization work, sequestration system infrastructure, and monitoring program to support permanent sequestration of the CO
2 captured at the plant.
In addition to individual carbon capture and sequestration projects, there are a number of U.S. programs designed to research, develop, and deploy CCS technologies on a broad scale. These include the National Energy Technology Laboratory's (NETL) Carbon Sequestration Program, regional carbon sequestration partnerships and the Carbon Sequestration Leadership Forum (CSLF).[101][102]
United Kingdom
The government of the United Kingdom has launched a tender process for a CCS demonstration project. The project will use post-combustion technology on coal-fired power generation at 300–400 megawatts or equivalent. The project aims to be operational by 2014.[103][104] The Government announced in June 2008 that four companies had prequalified for the following stages of the competition: BP Alternative Energy International Limited, EON UK Plc, Peel Power Limited and Scottish Power Generation Limited.[105] BP has subsequently withdrawn from the competition, claiming it could not find a power generator partner, and RWE npower is seeking a judicial review of the process after it did not qualify.[106]Doosan Babcock has modified their Clean Combustion Test Facility (CCTF) in Renfrew, Scotland to create the largest Oxyfuel test facility currently in the world.[citation needed] Oxyfuel firing on pulverized coal with recycled flue gas demonstrates the operation of a full scale 40 MW burner for use in coal-fired boilers. Sponsors of the project include the UK Department for Business Enterprise and Regulatory Reform (BERR,) as well as a group of industrial sponsors and university partners comprising Scottish and Southern Energy (Prime Sponsor), E.ON UK PLC, Drax Power Limited, ScottishPower, EDF Energy, Dong Energy Generation, Air Products Plc (Sponsors), and Imperial College and University of Nottingham (University Partners).[107]
In August 2010, clean energy new-comers B9 Coal announced their intention to join the competition with a CCS project in the North East of England. The proposal combines alkaline fuel cells with underground coal gasification for upwards of 90% carbon capture as a by-product. It is the only project of its kind to join the competition, using coal reserves in an environmentally friendly and efficient way.[citation needed] After costs increased to 13 billion pounds in 2011, the UK withdrew its support and ScottishPower cancelled its CCS project with Aker Clean Carbon.[citation needed]
In 2009 UK firm 2Co Energy was awarded planning permission for a £5bn power station and carbon-capture-and-storage project at Hatfield, near Doncaster and £164m of EU funding. Technology giant Samsung has agreed to take a 15% stake in the project.[108] It is planned to construct a 60 km (37 mi) pipeline from Stainforth, near Hatfield in South Yorkshire to Barmston in the East Riding of Yorkshire. CO
2 will then be stored in natural porous rock beneath the North Sea. National Grid believes the project has the potential to reduce CO
2 emissions from power stations across Yorkshire and the Humber by up to 90% with both the White Rose CCS project at Drax Power Station in North Yorkshire along with the proposed Don Valley Power Project at Hatfield benefiting from the scheme.[109][110][111]
In the Northeast of England, The Northeast of England Process Industry Cluster (NEPIC)of commodity chemical manufacturers are amongst the largest single point producers of carbon dioxide in the United Kingdom and they have created within NEPIC the Process Industry Carbon Capture and Storage Initiative[112] (PICCSI) to study the possibility of a carbon capture and storage (CCS) solution being provided for the chemical and steel manufacturing industry on Teesside, as well as for any carbon based energy production. This CCS technology option is being considered as a result of climate change regulations and the carbon taxation that could become a prohibitive cost for such energy intensive industries.
China
In Beijing, as of 2009, one major power plant is capturing and re-selling a small fraction of its CO2 emissions.[113]
Germany
The German industrial area of Schwarze Pumpe, about 4 kilometres (2.5 mi) south of the city of Spremberg, is home to the world's first CCS coal plant.[114] The mini pilot plant is run by an Alstom-built oxy-fuel boiler and is also equipped with a flue gas cleaning facility to remove fly ash and sulphur dioxide. The Swedish company Vattenfall AB invested some 70 million Euros in the two-year project, which began operation September 9, 2008. The power plant, which is rated at 30 megawatts, is a pilot project to serve as a prototype for future full-scale power plants.[115][116] 240 tonnes a day of CO2 are being trucked 350 kilometers (220 mi) where it will be injected into an empty gas field. Germany's BUND group called it a "fig leaf". For each tonne of coal burned, 3.6 tonnes of carbon dioxide is produced.[117] The CCS program at Schwarze Pump ended in 2014 due to unvialbe costs and energy use.[118]
German utility RWE operates a pilot-scale CO
2 scrubber at the lignite-fired Niederaußem power station built in cooperation with BASF (supplier of detergent) and Linde engineering.[119]
In Jänschwalde, Germany,[120] a plan is in the works for an Oxyfuel boiler, rated at 650 thermal MW (around 250 electric MW), which is about 20 times more than Vattenfall's 30 MW pilot plant under construction, and compares to today's largest Oxyfuel test rigs of 0.5 MW. Post-combustion capture technology will also be demonstrated at Jänschwalde.[121]
Australia
The Federal Resources and Energy Minister Martin Ferguson opened the first geosequestration project in the southern hemisphere in April 2008. The demonstration plant is near Nirranda South in South Western Victoria. ( 35°19′S 149°08′E / 35.31°S 149.14°E) The plant is owned by the Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC). CO2CRC is a non profit research collaboration supported by government and industry. The project has stored and monitored over 65,000 tonnes of carbon dioxide-rich gas which was extracted from a natural gas reservoir via a well, compressed and piped 2.25 km to a new well. There the gas has been injected into a depleted natural gas reservoir approximately two kilometers below the surface.[122][123] The project has moved to a second stage and is investigating carbon dioxide trapping in a saline aquifer 1500 meters below the surface. The Otway Project is a research and demonstration project, focused on comprehensive monitoring and verification.[124]This plant does not propose to capture CO
2 from coal-fired power generation, though two CO2CRC demonstration projects at a Victorian power station and research gasifier are demonstrating solvent, membrane, and adsorbent capture technologies from coal combustion.[125] Currently, only small-scale projects are storing CO
2 stripped from the products of combustion of coal burnt for electricity generation at coal-fired power stations.[126] Work currently being carried out by the GreenMag Group and the University of Newcastle and funded by the New South Wales and Australian Governments and industry intends to have a working mineral carbonation pilot plant in operation by 2013.[34]
View the full list of Zero Emission Projects for fossil fuel power plant in Europe.[127]
Limitations of CCS for power stations
Critics say large-scale CCS deployment is unproven and decades away from being commercialized. They say that it is risky and expensive and that a better option is renewable energy. Some environmental groups point out that CCS technology leaves behind dangerous waste material that has to be stored, just like nuclear power stations.[128]Another limitation of CCS is its energy penalty. The technology is expected to use between 10 and 40 percent of the energy produced by a power station.[129] Wide-scale adoption of CCS may erase efficiency gains in coal power plants of the last 50 years, and increase resource consumption by one third. Even taking the fuel penalty into account, however, overall levels of CO
2 abatement would remain high at approximately 80–90%, compared to a plant without CCS.[130] It is possible for CCS, when combined with biomass, to result in net negative emissions.[131] Though, all of the currently (as of Feb 2011) operational BECCS (Bio-energy with carbon capture and storage) plants operate on point emissions other than power stations, such as biofuel refineries.[132]
The use of CCS can reduce CO
2 emissions from the stacks of coal power plants by 85–90% or more, but it has no effect on CO
2 emissions due to the mining and transport of coal. It will actually "increase such emissions and of air pollutants per unit of net delivered power and will increase all ecological, land-use, air-pollution, and water-pollution impacts from coal mining, transport, and processing, because the CCS system requires 25% more energy, thus 25% more coal combustion, than does a system without CCS".[133]
Another concern regards the permanence of storage schemes. Opponents to CCS claim that safe and permanent storage of CO
2 cannot be guaranteed and that even very low leakage rates could undermine any climate mitigation effect.[129] In 1986 a large leakage of naturally sequestered CO
2 rose from Lake Nyos in Cameroon and asphyxiated 1,700 people. While the carbon had been sequestered naturally, some point to the event as evidence for the potentially catastrophic effects of sequestering carbon artificially.[49][128]
On one hand, Greenpeace claims that CCS could lead to a doubling of coal plant costs.[129] It is also claimed by opponents to CCS that money spent on CCS will divert investments away from other solutions to climate change. On the other hand, CCS is pointed out as economically attractive in comparison to other forms of low carbon electricity generation and seen by the IPCC and others as a critical component for meeting mitigation targets such as 450 ppm and 350 ppm.[134][135][136]
Cost
Although the processes involved in CCS have been demonstrated in other industrial applications, no commercial scale projects which integrate these processes exist; the costs therefore are somewhat uncertain. Some recent credible estimates indicate that the cost of capturing and storing carbon dioxide is US$60 per ton,[137] corresponding to an increase in electricity prices of about US 6c per kWh (based on typical coal-fired power plant emissions of 2.13 pounds CO2 per kWh). This would double the typical US industrial electricity price (now at around 6c per kWh) and increase the typical retail residential electricity price by about 50% (assuming 100% of power is from coal, which may not necessarily be the case, as this varies from state to state). Similar (approximate) price increases would likely be expected in coal dependent countries such as Australia, because the capture technology and chemistry, as well as the transport and injection costs from such power plants would not, in an overall sense, vary significantly from country to country.[citation needed]
The reasons that CCS is expected to cause such power price increases are several. Firstly, the increased energy requirements of capturing and compressing CO
2 significantly raises the operating costs of CCS-equipped power plants. In addition, there are added investment and capital costs. The process would increase the fuel requirement of a plant with CCS by about 25% for a coal-fired plant, and about 15% for a gas-fired plant.[4] The cost of this extra fuel, as well as storage and other system costs, are estimated to increase the costs of energy from a power plant with CCS by 30–60%, depending on the specific circumstances. Pre-commercial CCS demonstration projects are likely to be more expensive than mature CCS technology; the total additional costs of an early large-scale CCS demonstration project are estimated to be €0.5-1.1 billion per project over the project lifetime. Other applications are possible. In the belief that use of sequestered carbon could be harnessed to offset the cost of capture and storage, Walker Architects published the first CO
2 gas CAES application, proposing the use of sequestered CO
2 for Energy Storage on October 24, 2008. To date the feasibility of such potential offsets to the cost have not been examined.[138]
| Natural gas combined cycle | Pulverized coal | Integrated gasification combined cycle | ||||
| Without capture (reference plant) | 0.03–0.05 | 0.04–0.05 | 0.04–0.06 | |||
| With capture and geological storage | 0.04–0.08 | 0.06–0.10 | 0.06–0.09 | |||
| (Cost of capture and geological storage) | 0.01–0.03 | 0.02–0.05 | 0.02–0.03 | |||
| With capture and Enhanced oil recovery | 0.04–0.07 | 0.05–0.08 | 0.04–0.08 | |||
All costs refer to costs for energy from newly built, large-scale plants. Natural gas combined cycle costs are based on natural gas prices of US$2.80–4.40 per GJ (LHV based). Energy costs for PC and IGCC are based on bituminous coal costs of US$1.00–1.50 per GJ LHV. Note that the costs are very dependent on fuel prices (which change continuously), in addition to other factors such as capital costs. Also note that for EOR, the savings are greater for higher oil prices. Current gas and oil prices are substantially higher than the figures used here. All figures in the table are from Table 8.3a in [IPCC, 2005].[4] |
||||||
The cost of CCS depends on the cost of capture and storage, which varies according to the method used. Geological storage in saline formations or depleted oil or gas fields typically cost US$0.50–8.00 per tonne of CO
2 injected, plus an additional US$0.10–0.30 for monitoring costs. When storage is combined with enhanced oil recovery to extract extra oil from an oil field, however, the storage could yield net benefits of US$10–16 per tonne of CO
2 injected (based on 2003 oil prices). This would likely negate some of the effect of the carbon capture when the oil was burnt as fuel. Even taking this into account, as the table above shows, the benefits do not outweigh the extra costs of capture.[citation needed]
Cost of electricity generated by different sources including those incorporating CCS technologies can be found in cost of electricity by source. If CO
2 capture was part of a fuel cycle then the CO
2 would have value rather than be a cost. The proposed Solar Fuel or methane cycle proposed by the Fraunhofer Society[citation needed] amongst others is an example. This "solar fuel"[139] cycle uses the excess electrical renewable energy to create hydrogen via electrolysis of water.[140][141] The hydrogen is then combined with CO
2 to create synthetic natural gas SNG and stored in the gas network. See the latest Cost Report on the Cost of CO
2 Capture produced by the Zero Emissions Platform
Governments around the world have provided a range of different types of funding support to CCS demonstration projects, including tax credits, allocations and grants. The funding is associated with both a desire to accelerate innovation activities for CCS as a low-carbon technology and the need for economic stimulus activities. As of 2011, approximately US$23.5bn has been made available to support large-scale CCS demonstration projects around the world.[142]
Carbon Capture and Storage and the Kyoto Protocol
One way to finance future CCS projects could be through the Clean Development Mechanism of the Kyoto Protocol. At COP16 in 2010, The Subsidiary Body for Scientific and Technological Advice, at its thirty-third session, issued a draft document recommending the inclusion of Carbon dioxide capture and storage in geological formations in Clean Development Mechanism project activities.[143] At COP17 in Durban, a final agreement was reached enabling CCS projects to receive support through the Clean Development Mechanism.[144]Environmental effects
The theoretical merit of CCS systems is the reduction of CO2 emissions by up to 90%, depending on plant type. Generally, environmental effects from use of CCS arise during power production, CO
2 capture, transport, and storage. Issues relating to storage are discussed in those sections.
Additional energy is required for CO
2 capture, and this means that substantially more fuel has to be used to produce the same amount of power, depending on the plant type. For new super-critical pulverized coal (PC) plants using current technology, the extra energy requirements range from 24 to 40%, while for natural gas combined cycle (NGCC) plants the range is 11–22% and for coal-based gasification combined cycle (IGCC) systems it is 14–25% [IPCC, 2005].[145] Obviously, fuel use and environmental problems arising from mining and extraction of coal or gas increase accordingly. Plants equipped with flue-gas desulfurization (FGD) systems for sulfur dioxide control require proportionally greater amounts of limestone, and systems equipped with selective catalytic reduction systems for nitrogen oxides produced during combustion require proportionally greater amounts of ammonia.
IPCC has provided estimates of air emissions from various CCS plant designs (see table below). While CO
2 is drastically reduced though never completely captured, emissions of air pollutants increase significantly, generally due to the energy penalty of capture. Hence, the use of CCS entails a reduction in air quality. Type and amount of air pollutants still depends on technology. CO
2 is captured with alkaline solvents catching the acidic CO
2 at low temperatures in the absorber and releasing CO
2 at higher temperatures in a desorber. Chilled Ammonia CCS Plants have inevitable ammonia emissions to air. "Functionalized Ammonia" emit less ammonia, but amines may form secondary amines and these will emit volatile nitrosamines[146] by a side reaction with nitrogendioxide, which is present in any flue gas even after DeNOx. Nevertheless, there are advanced amines in testing with little to no vapor pressure to avoid these amine- and consecutive nitrosamine emissions. Nevertheless, all the capture plants amines have in common, that practically 100% of remaining sulfur dioxide from the plant is washed out of the flue gas, the same applies to dust/ash.
| Natural gas combined cycle | Pulverized coal | Integrated gasification combined cycle | ||||
| CO 2 |
43 (-89%) | 107 (−87%) | 97 (−88%) | |||
| NOX | 0.11 (+22%) | 0.77 (+31%) | 0.1 (+11%) | |||
| SOX | - | 0.001 (−99.7%) | 0.33 (+17.9%) | |||
| Ammonia | 0.002 (before: 0) | 0.23 (+2200%) | - | |||
Based on Table 3.5 in [IPCC, 2005]. Between brackets the increase or decrease compared to a similar plant without CCS. |
||||||

 WikiMiniAtlas
WikiMiniAtlas