| Photoreceptor cell | |
|---|---|
| |
| Identifiers | |
| MeSH | D010786 |
A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light (visible electromagnetic radiation) into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.
There are currently three known types of photoreceptor cells in mammalian eyes: rods, cones, and intrinsically photosensitive retinal ganglion cells. The two classic photoreceptor cells are rods and cones, each contributing information used by the visual system to form a representation of the visual world, sight.
The rods are narrower than the cones and distributed differently across
the retina, but the chemical process in each that supports
phototransduction is similar. A third class of mammalian photoreceptor cell was discovered during the 1990s:
the intrinsically photosensitive retinal ganglion cells. These cells do
not contribute to sight directly, but are thought to support circadian rhythms and pupillary reflex.
There are major functional differences between the rods and
cones. Rods are extremely sensitive, and can be triggered by a single
photon. At very low light levels, visual experience is based solely on the rod signal.
Cones require significantly brighter light (that is, a larger
number of photons) to produce a signal. In humans, there are three
different types of cone cell, distinguished by their pattern of response
to light of different wavelengths. Color experience is calculated from
these three distinct signals, perhaps via an opponent process.
This explains why colors cannot be seen at low light levels, when only
the rod and not the cone photoreceptor cells are active. The three types
of cone cell respond (roughly) to light of short, medium, and long
wavelengths, so they may respectively be referred to as S-cones,
M-cones, and L-cones.
In accordance with the principle of univariance,
the firing of the cell depends upon only the number of photons
absorbed. The different responses of the three types of cone cells are
determined by the likelihoods that their respective photoreceptor
proteins will absorb photons of different wavelengths. So, for example,
an L cone cell contains a photoreceptor protein that more readily
absorbs long wavelengths of light (that is, more "red"). Light of a
shorter wavelength can also produce the same response from an L cone
cell, but it must be much brighter to do so.
The human retina contains about 120 million rod cells, and 6
million cone cells. The number and ratio of rods to cones varies among
species, dependent on whether an animal is primarily diurnal or nocturnal. Certain owls, such as the nocturnal tawny owl,
have a tremendous number of rods in their retinae. In the human visual
system, in addition to the photosensitive rods & cones, there are
about 2.4 million to 3 million ganglion cells, with 1 to 2% of them being photosensitive. The axons of ganglion cells form the two optic nerves.
Photoreceptor cells are typically arranged in an irregular but approximately hexagonal grid, known as the retinal mosaic.
The pineal and parapineal glands are photoreceptive in
non-mammalian vertebrates, but not in mammals. Birds have photoactive
cerebrospinal fluid (CSF)-contacting neurons within the paraventricular
organ that respond to light in the absence of input from the eyes or
neurotransmitters. Invertebrate photoreceptors in organisms such as insects and molluscs are different in both their morphological organization and their underlying biochemical pathways. This article describes human photoreceptors.
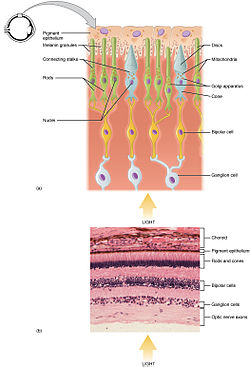
Histology
Rod and cone photoreceptors are found on the outermost layer of the retina; they both have the same basic structure. Closest to the visual field (and farthest from the brain) is the axon terminal, which releases a neurotransmitter called glutamate to bipolar cells. Farther back is the cell body, which contains the cell's organelles. Farther back still is the inner segment, a specialized part of the cell full of mitochondria. The chief function of the inner segment is to provide ATP (energy) for the sodium-potassium pump. Finally, closest to the brain (and farthest from the field of view) is the outer segment, the part of the photoreceptor that absorbs light. Outer segments are actually modified cilia that contain disks filled with opsin, the molecule that absorbs photons, as well as voltage-gated sodium channels.
The membranous photoreceptor protein opsin contains a pigment molecule called retinal. In rod cells, these together are called rhodopsin. In cone cells, there are different types of opsins that combine with retinal to form pigments called photopsins.
Three different classes of photopsins in the cones react to different
ranges of light frequency, a differentiation that allows the visual
system to calculate color. The function of the photoreceptor cell is to
convert the light energy of the photon into a form of energy
communicable to the nervous system and readily usable to the organism:
This conversion is called signal transduction.
The opsin found in the intrinsically photosensitive ganglion cells of the retina is called melanopsin.
These cells are involved in various reflexive responses of the brain
and body to the presence of (day)light, such as the regulation of circadian rhythms, pupillary reflex and other non-visual responses to light. Melanopsin functionally resembles invertebrate opsins.
When light activates the melanopsin signaling system, the melanopsin-containing ganglion cells discharge nerve impulses that are conducted through their axons to specific brain targets. These targets include the olivary pretectal nucleus (a center responsible for controlling the pupil of the eye), the LGN, and, through the retinohypothalamic tract (RHT), the suprachiasmatic nucleus
of the hypothalamus (the master pacemaker of circadian rhythms).
Melanopsin-containing ganglion cells are thought to influence these
targets by releasing from their axon terminals the neurotransmitters glutamate and pituitary adenylate cyclase activating polypeptide (PACAP).
Humans
Normalized human photoreceptor absorbances for different wavelengths of light
Illustration
of the distribution of cone cells in the fovea of an individual with
normal color vision (left), and a color blind (protanopic) retina. Note
that the center of the fovea holds very few blue-sensitive cones.
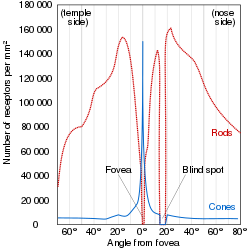
Distribution of rods and cones along a line passing through the fovea and the blind spot of a human eye
The human retina has approximately 6 million cones and 120 million rods. Signals from the rods and cones converge on ganglion and bipolar cells for preprocessing before they are sent to the lateral geniculate nucleus. At the "center" of the retina (the point directly behind the lens) lies the fovea (or fovea centralis), which contains only cone cells; and is the region capable of producing the highest visual acuity or highest resolution. Across the rest of the retina, rods and cones are intermingled. No photoreceptors are found at the blind spot, the area where ganglion cell fibers are collected into the optic nerve and leave the eye.
The photoreceptor proteins in the three types of cones differ in their sensitivity to photons of different wavelengths
(see graph). Since cones respond to both the wavelength and intensity
of light, the cone's sensitivity to wavelength is measured in terms of
its relative rate of response if the intensity of a stimulus is held
fixed, while the wavelength is varied. From this, in turn, is inferred
the absorbance.
The graph normalizes the degree of absorbance on a hundred-point scale.
For example, the S cone's relative response peaks around 420 nm
(nanometers, a measure of wavelength). This tells us that an S cone is
more likely to absorb a photon at 420 nm than at any other wavelength.
If light of a different wavelength to which it is less sensitive, say
480 nm, is increased in brightness appropriately, however, it will
produce exactly the same response in the S cone. So, the colors of the
curves are misleading. Cones cannot detect color by themselves; rather,
color vision requires comparison of the signal across different cone types.
Phototransduction
The process of phototransduction occurs in the retina. The retina has many layers of various cell types. The most numerous photoreceptor cells (rods and cones)
form the outermost layer. These are the photoreceptors responsible for
mediating the sense sight. The middle retinal layer contains bipolar
cells, collect signals from photoreceptors and transmit them to the
retinal ganglion cells of the innermost retinal layer. Retinal ganglion
cell axons collectively form the optic nerve, via which they project to the brain.
Unlike most sensory receptor cells, photoreceptors actually become hyperpolarized when stimulated; and conversely are depolarized
when not stimulated. This means that glutamate is released continuously
when the cell is unstimulated, and stimulus causes release to stop. In
the dark, cells have a relatively high concentration of cyclic guanosine 3'-5' monophosphate (cGMP), which opens cGMP-gated ion channels.
These channels are nonspecific, allowing movement of both sodium and
calcium ions when open. The movement of these positively charged ions
into the cell (driven by their respective electrochemical gradient) depolarizes the membrane, and leads to the release of the neurotransmitter glutamate.
When light hits a photoreceptive pigment within the photoreceptor
cell, the pigment changes shape. The pigment, called iodopsin or
rhodopsin, consists of large proteins called opsin (situated in the
plasma membrane), attached to a covalently bound prosthetic group: an
organic molecule called retinal (a derivative of vitamin A). The retinal
exists in the 11-cis-retinal form when in the dark, and stimulation by
light causes its structure to change to all-trans-retinal. This
structural change causes opsin (a G protein-coupled receptor) to activate its G protein transducin, which leads to the activation of cGMP phosphodiesterase,
which breaks cGMP down into 5'-GMP. Reduction in cGMP allows the ion
channels to close, preventing the influx of positive ions,
hyperpolarizing the cell, and stopping the release of neurotransmitters. The entire process by which light initiates a sensory response is called visual phototransduction.
Dark current
Unstimulated (in the dark), cyclic-nucleotide gated channels in the outer segment are open because cyclic GMP (cGMP) is bound to them. Hence, positively charged ions (namely sodium ions) enter the photoreceptor, depolarizing it to about −40 mV (resting potential in other nerve cells is usually −65 mV). This depolarization current is often known as dark current.
Signal transduction pathway
The absorption of light leads to an isomeric change in the retinal molecule.
The signal transduction
pathway is the mechanism by which the energy of a photon signals a
mechanism in the cell that leads to its electrical polarization. This
polarization ultimately leads to either the transmittance or inhibition
of a neural signal that will be fed to the brain via the optic nerve. The steps, or signal transduction pathway, in the vertebrate eye's rod and cone photoreceptors are then:
- The rhodopsin or iodopsin in the disc membrane of the outer segment absorbs a photon, changing the configuration of a retinal Schiff base cofactor inside the protein from the cis-form to the trans-form, causing the retinal to change shape.
- This results in a series of unstable intermediates, the last of which binds stronger to a G protein in the membrane, called transducin, and activates it. This is the first amplification step – each photoactivated rhodopsin triggers activation of about 100 transducins.
- Each transducin then activates the enzyme cGMP-specific phosphodiesterase (PDE).
- PDE then catalyzes the hydrolysis of cGMP to 5' GMP. This is the second amplification step, where a single PDE hydrolyses about 1000 cGMP molecules.
- The net concentration of intracellular cGMP is reduced (due to its conversion to 5' GMP via PDE), resulting in the closure of cyclic nucleotide-gated Na+ ion channels located in the photoreceptor outer segment membrane.
- As a result, sodium ions can no longer enter the cell, and the photoreceptor outer segment membrane becomes hyperpolarized, due to the charge inside the membrane becoming more negative.
- This change in the cell's membrane potential causes voltage-gated calcium channels to close. This leads to a decrease in the influx of calcium ions into the cell and thus the intracellular calcium ion concentration falls.
- A decrease in the intracellular calcium concentration means that less glutamate is released via calcium-induced exocytosis to the bipolar cell (see below). (The decreased calcium level slows the release of the neurotransmitter glutamate, which excites the postsynaptic bipolar cells and horizontal cells.)
- Reduction in the release of glutamate means one population of bipolar cells will be depolarized and a separate population of bipolar cells will be hyperpolarized, depending on the nature of receptors (ionotropic or metabotropic) in the postsynaptic terminal.
Thus, a rod or cone photoreceptor actually releases less
neurotransmitter when stimulated by light. Less neurotransmitter in the
synaptic cleft between a photoreceptor and bipolar cell will serve to
either excite (depolarize) ON bipolar cells or inhibit (hyperpolarize)
OFF bipolar cells. Thus, it is at the photoreceptor-bipolar cell synapse
where visual signals are split into ON and OFF pathways.
ATP provided by the inner segment powers the sodium-potassium
pump. This pump is necessary to reset the initial state of the outer
segment by taking the sodium ions that are entering the cell and pumping
them back out.
Although photoreceptors are neurons, they do not conduct action potentials with the exception of the photosensitive ganglion cell – which are involved mainly in the regulation of circadian rhythms, melatonin, and pupil dilation.
Advantages
Phototransduction in rods and cones is somewhat unusual in that the stimulus
(in this case, light) reduces the cell's response or firing rate,
different from most other sensory systems in which a stimulus increases
the cell's response or firing rate. This difference has important
functional consequences:
First, the classic (rod or cone) photoreceptor is depolarized in
the dark, which means many sodium ions are flowing into the cell. Thus,
the random opening or closing of sodium channels will not affect the
membrane potential of the cell; only the closing of a large number of
channels, through absorption of a photon, will affect it and signal that
light is in the visual field. This system may have less noise relative
to sensory transduction schema that increase rate of neural firing in
response to stimulus, like touch and olfaction.
Second, there is a lot of amplification in two stages of classic phototransduction: one pigment will activate many molecules of transducin,
and one PDE will cleave many cGMPs. This amplification means that even
the absorption of one photon will affect membrane potential and signal
to the brain that light is in the visual field. This is the main feature
that differentiates rod photoreceptors from cone photoreceptors. Rods
are extremely sensitive and have the capacity of registering a single
photon of light, unlike cones. On the other hand, cones are known to
have very fast kinetics in terms of rate of amplification of
phototransduction, unlike rods.
Difference between rods and cones
Comparison of human rod and cone cells, from Eric Kandel et al. in Principles of Neural Science.
| Rods | Cones |
|---|---|
| Used for scotopic vision (vision under low light conditions) | Used for photopic vision (vision under high light conditions) |
| Very light sensitive; sensitive to scattered light | Not very light sensitive; sensitive only to direct light |
| Loss causes night blindness | Loss causes legal blindness |
| Low visual acuity | High visual acuity; better spatial resolution |
| Not present in fovea | Concentrated in fovea |
| Slow response to light, stimuli added over time | Fast response to light, can perceive more rapid changes in stimuli |
| Have more pigment than cones, so can detect lower light levels | Have less pigment than rods, require more light to detect images |
| Stacks of membrane-enclosed disks are unattached to cell membrane directly | Disks are attached to outer membrane |
| About 120 million rods distributed around the retina | About 6 million cones distributed in each retina |
| One type of photosensitive pigment | Three types of photosensitive pigment in humans |
| Confer achromatic vision | Confer color vision |
Function
A given photoreceptor responds to both the wavelength and intensity
of a light source. For example, red light at a certain intensity can
produce the same exact response in a photoreceptor as the green light of
different intensity. Therefore, the response of a single photoreceptor
is ambiguous when it comes to color.
Development
The
key events mediating rod versus S cone versus M cone differentiation
are induced by several transcription factors, including RORbeta, OTX2,
NRL, CRX, NR2E3 and TRbeta2. The S cone fate represents the default
photoreceptor program, however differential transcriptional activity can
bring about rod or M cone generation. L cones are present in primates,
however there is not much known for their developmental program due to
use of rodents in research. There are five steps to developing
photoreceptors: proliferation of multi-potent retinal progenitor cells
(RPCs); restriction of competence of RPCs; cell fate specification;
photoreceptor gene expression; and lastly axonal growth, synapse
formation and outer segment growth.
Early Notch
signaling maintains progenitor cycling. Photoreceptor precursors come
about through inhibition of Notch signaling and increased activity of
various factors including achaete-scute homologue 1. OTX2 activity
commits cells to the photoreceptor fate. CRX further defines the
photoreceptor specific panel of genes being expressed. NRL expression
leads to the rod fate. NR2E3 further restricts cells to the rod fate by
repressing cone genes. RORbeta is needed for both rod and cone
development. TRbeta2 mediates the M cone fate. If any of the
previously mentioned factors' functions are ablated, the default
photoreceptor is a S cone. These events take place at different time
periods for different species and include a complex pattern of
activities that bring about a spectrum of phenotypes. If these
regulatory networks are disrupted, retinitis pigmentosa, macular degeneration or other visual deficits may result.
Signaling
3D medical illustration of the rod and cone structure of photoreceptors.
The rod and cone photoreceptors signal their absorption of photons
via a decrease in the release of the neurotransmitter glutamate to
bipolar cells at its axon terminal. Since the photoreceptor is
depolarized in the dark, a high amount of glutamate is being released to
bipolar cells in the dark. Absorption of a photon will hyperpolarize
the photoreceptor and therefore result in the release of less glutamate at the presynaptic terminal to the bipolar cell.
Every rod or cone photoreceptor releases the same
neurotransmitter, glutamate. However, the effect of glutamate differs in
the bipolar cells, depending upon the type of receptor imbedded in that cell's membrane. When glutamate binds to an ionotropic receptor,
the bipolar cell will depolarize (and therefore will hyperpolarize with
light as less glutamate is released). On the other hand, binding of
glutamate to a metabotropic receptor results in a hyperpolarization, so this bipolar cell will depolarize to light as less glutamate is released.
In essence, this property allows for one population of bipolar
cells that gets excited by light and another population that gets
inhibited by it, even though all photoreceptors show the same response
to light. This complexity becomes both important and necessary for
detecting color, contrast, edges, etc.
Further complexity arises from the various interconnections among bipolar cells, horizontal cells, and amacrine cells
in the retina. The final result is differing populations of ganglion
cells in the retina, a sub-population of which is also intrinsically
photosensitive, using the photopigment melanopsin.
Ganglion cell (non-rod non-cone) photoreceptors
A non-rod non-cone photoreceptor in the eyes of mice, which was shown to mediate circadian rhythms, was discovered in 1991 by Foster et al. These neuronal cells, called intrinsically photosensitive retinal ganglion cells (ipRGC), are a small subset (≈1–3%) of the retinal ganglion cells located in the inner retina, that is, in front of the rods and cones located in the outer retina. These light sensitive neurons contain a photopigment, melanopsin, which has an absorption peak of the light at a different wavelength (≈480 nm) than rods and cones. Beside circadian / behavioral functions, ipRGCs have a role in initiating the pupillary light reflex.
Dennis Dacey with colleagues showed in a species of Old World
monkey that giant ganglion cells expressing melanopsin projected to the
lateral geniculate nucleus (LGN).
Previously only projections to the midbrain (pre-tectal nucleus) and
hypothalamus (suprachiasmatic nucleus) had been shown. However a visual
role for the receptor was still unsuspected and unproven.
In 2007, Farhan H. Zaidi and colleagues published pioneering work using rodless coneless humans. Current Biology
subsequently announced in their 2008 editorial, commentary and
despatches to scientists and ophthalmologists, that the non-rod non-cone
photoreceptor had been conclusively discovered in humans using landmark
experiments on rodless coneless humans by Zaidi and colleagues
As had been found in other mammals, the identity of the non-rod
non-cone photoreceptor in humans was found to be a ganglion cell in the
inner retina. The workers had tracked down patients with rare diseases
wiping out classic rod and cone photoreceptor function but preserving
ganglion cell function.
Despite having no rods or cones the patients continued to exhibit
circadian photoentrainment, circadian behavioural patterns, melanopsin
suppression, and pupil reactions, with peak spectral sensitivities to
environmental and experimental light matching that for the melanopsin
photopigment. Their brains could also associate vision with light of
this frequency.
In humans the retinal ganglion cell photoreceptor contributes to conscious sight as well as to non-image-forming functions like circadian rhythms, behaviour and pupil reactions. Since these cells respond mostly to blue light, it has been suggested that they have a role in mesopic vision.
Zaidi and colleagues' work with rodless coneless human subjects hence
also opened the door into image-forming (visual) roles for the ganglion
cell photoreceptor. It was discovered that there are parallel pathways
for vision – one classic rod and cone-based pathway arising from the
outer retina, and the other a rudimentary visual brightness detector
pathway arising from the inner retina, which seems to be activated by
light before the other.
Classic photoreceptors also feed into the novel photoreceptor system,
and color constancy may be an important role as suggested by Foster. The
receptor could be instrumental in understanding many diseases including
major causes of blindness worldwide like glaucoma, a disease that
affects ganglion cells, and the study of the receptor offered potential
as a new avenue to explore in trying to find treatments for blindness.
It is in these discoveries of the novel photoreceptor in humans and in
the receptor's role in vision, rather than its non-image-forming
functions, where the receptor may have the greatest impact on society as
a whole, though the impact of disturbed circadian rhythms is another
area of relevance to clinical medicine.
Most work suggests that the peak spectral sensitivity of the
receptor is between 460 and 482 nm. Steven Lockley et al. in 2003 showed
that 460 nm wavelengths of light suppress melatonin twice as much as
longer 555 nm light. However, in more recent work by Farhan Zaidi et
al., using rodless coneless humans, it was found that what consciously
led to light perception was a very intense 481 nm stimulus; this means
that the receptor, in visual terms, enables some rudimentary vision
maximally for blue light.
![Anatomy of a Rod Cell[8]](https://upload.wikimedia.org/wikipedia/commons/thumb/b/bb/Rod%26Cone.jpg/179px-Rod%26Cone.jpg)