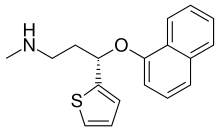
From Wikipedia, the free encyclopedia
Duloxetine, sold under the brand name
Cymbalta among others, is a medication used to treat
major depressive disorder,
generalized anxiety disorder,
fibromyalgia, and
neuropathic pain. It is taken by mouth.
Common side effects include dry mouth, nausea, feeling tired, dizziness, agitation, sexual problems, and increased sweating. Severe side effects include an increased risk of
suicide,
serotonin syndrome,
mania, and
liver problems.
Antidepressant withdrawal syndrome may occur if stopped. There are concerns that use during the later part of
pregnancy can harm the baby. It is a
serotonin–norepinephrine reuptake inhibitor. How it works is not entirely clear.
Duloxetine was approved for medical use in the United States in 2004. It is available as a
generic medication. In the United States the wholesale cost per dose is about 0.20 USD as of 2018. In 2016 it was the 48th most prescribed medication in the United States with more than 15 million prescriptions.
Medical uses
A 2014
Cochrane
review concluded that duloxetine is beneficial in the treatment of
diabetic neuropathy and fibromyalgia but that more comparative studies
with other medicines are needed. The French medical journal
Prescrire concluded that duloxetine is no better than other available agents and has a greater risk of side effects. Thus they recommend against its general use.
Major depressive disorder
Duloxetine
was approved for the treatment of major depression in 2004. While
duloxetine has demonstrated improvement in depression-related symptoms
compared to
placebo,
comparisons of duloxetine to other antidepressant medications have been
less successful. A 2012 Cochrane Review did not find greater efficacy
of duloxetine compared to SSRIs and newer antidepressants. Additionally,
the review found evidence that duloxetine has increased side effects
and reduced tolerability compared to other antidepressants. It thus did
not recommend duloxetine as a first line treatment for major depressive
disorder, given the (then) high cost of duloxetine compared to
inexpensive off-patent antidepressants and lack of increased efficacy. Duloxetine appears less tolerable than some other antidepressants. Generic duloxetine became available in 2013.
Generalized anxiety disorder
Diabetic neuropathy
Duloxetine was approved for the pain associated with diabetic
peripheral neuropathy
(DPN), based on the positive results of two clinical trials. The
average daily pain was measured using an 11-point scale, and duloxetine
treatment resulted in an additional 1–1.7 points decrease of pain as
compared with placebo.
At least 50% pain relief was achieved in 40–45% of the duloxetine
patients vs. 20–22% of placebo patients. Pain decreased by more than
90%, in 9–14% of duloxetine patients vs. 2–4% of placebo patients. Most
of the response was achieved in the first two weeks on the medication.
Duloxetine slightly increased the fasting
serum glucose; this effect was deemed to be of "minimal clinical significance", however.
The comparative efficacy of duloxetine and established
pain-relief medications for DPN is unclear. A systematic review noted
that
tricyclic antidepressants (
imipramine and
amitriptyline), traditional
anticonvulsants and
opioids
have better efficacy than duloxetine. Duloxetine, tricyclic
antidepressants and anticonvulsants have similar tolerability while the
opioids caused more side effects. Another review in
Prescrire International
considered the moderate pain relief achieved with duloxetine to be
clinically insignificant and the results of the clinical trials
unconvincing. The reviewer saw no reason to prescribe duloxetine in
practice. The comparative data collected by reviewers in
BMC Neurology
indicated that amitriptyline, other tricyclic antidepressants and
venlafaxine may be more effective. The authors noted that the evidence
in favor of duloxetine is much more solid, however.
A Cochrane review concluded that the evidence in support of
duloxetine's efficacy in treating painful diabetic neuropathy was
adequate, and that further trials should focus on comparisons with other
medications.
Fibromyalgia and chronic pain
A review of duloxetine found that it reduced pain and fatigue, and improved physical and mental performance compared to placebo.
On November 4, 2010, the U.S. Food and Drug Administration
approved duloxetine to treat chronic musculoskeletal pain, including
discomfort from osteoarthritis and chronic lower back pain.
Stress urinary incontinence
Duloxetine failed to receive US approval for
stress urinary incontinence amid concerns over liver toxicity and suicidal events; it was approved for this use in the
UK, however, where it is recommended as an add-on medication in stress urinary incontinence instead of surgery.
The safety and utility of duloxetine in the treatment of
incontinence has been evaluated in a series of meta analyses and
practice guidelines.
- A 2017 meta-analysis found that harms are at least as great if not greater than the benefits.
- A 2013 meta-analysis
concluded that duloxetine decreased incontinence episodes more than
placebo with people about 56% more likely than placebo to experience a
50% decrease in episodes. Adverse effects were experienced by 83% of
duloxetine-treated subjects and by 45% of placebo-treated subjects.
- A 2012 review and practice guideline published by the European Association of Urology
concluded that the clinical trial data provides Grade 1a evidence that
duloxetine improves but does not cure urinary incontinence, and that it
causes a high rate of gastrointestinal side effects (mainly nausea and
vomiting) leading to a high rate of treatment discontinuation.
- The National Institute for Clinical and Health Excellence recommends
(as of September 2013) that duloxetine not be routinely offered as
first line treatment, and that it only be offered as second line therapy
in women wishing to avoid therapy. The guideline further states that
women should be counseled regarding the drug's side effects.
Contraindications
- Hypersensitivity: duloxetine is contraindicated in patients with
a known hypersensitivity to duloxetine or any of the inactive
ingredients.
- Monoamine oxidase inhibitors (MAOIs): concomitant use in patients taking MAOIs is contraindicated.
- Uncontrolled narrow-angle glaucoma: in clinical trials, Cymbalta use was associated with an increased risk of mydriasis (dilation of the pupil); therefore, its use should be avoided in patients with uncontrolled narrow-angle glaucoma, in which mydriasis can cause sudden worsening.
- Central nervous system
(CNS) acting drugs: given the primary CNS effects of duloxetine, it
should be used with caution when it is taken in combination with or
substituted for other centrally acting drugs, including those with a
similar mechanism of action.
- Duloxetine and thioridazine should not be co-administered.
In addition, the FDA has reported on life-threatening drug
interactions that may be possible when co-administered with triptans and
other drugs acting on serotonin pathways leading to increased risk for
serotonin syndrome.
Adverse effects
In a trial for major depressive disorder (MDD), the most commonly
reported treatment-emergent adverse events among duloxetine-treated
patients were
nausea (34.7%),
dry mouth (22.7%),
headache (20.0%) and
dizziness (18.7%), and except for
headache, these were reported significantly more often than in the placebo group.
In a long-term study of fibromyalgia patients receiving duloxetine,
frequency and type of adverse effects was similar to that reported in
the MDD above. Side effects tended to be mild-to-moderate, and tended to
decrease in intensity over time.
In 4 clinical trials of duloxetine for the treatment of MDD,
sexual dysfunction
occurred significantly more frequently in patients treated with
duloxetine than those treated with placebo, and this difference occurred
only in men. Specifically, common side effects include difficulty becoming aroused, lack of interest in sex, and
anorgasmia (trouble achieving orgasm). Loss of or decreased response to sexual stimuli and ejaculatory
anhedonia are also reported.
Frequency of treatment-emergent sexual dysfunction were similar for
duloxetine and SSRIs when compared in a 6 month observational study in
depressed patients. Rates of sexual dysfunction in MDD patients treated with duloxetine vs
escitalopram
did not differ significantly at 4, 8, and 12 weeks of treatment,
although the trend favored duloxetine (33.3% of duloxetine patients
experienced sexual side effects compared to 43.6% of those receiving
escitalopram and 25% of those receiving placebo).
Discontinuation syndrome
During marketing of other SSRIs and SNRIs, there have been
spontaneous reports of adverse events occurring upon discontinuation of
these drugs, particularly when abrupt, including the following:
dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g.,
paresthesias such as
brain zap electric shock sensations), anxiety, confusion, headache, lethargy,
emotional lability,
insomnia,
hypomania,
tinnitus, and seizures. The
withdrawal syndrome from duloxetine resembles the
SSRI discontinuation syndrome.
When discontinuing treatment with duloxetine, the manufacturer
recommends a gradual reduction in the dose, rather than abrupt
cessation, whenever possible. If intolerable symptoms occur following a
decrease in the dose or upon discontinuation of treatment, then resuming
the previously prescribed dose may be considered. Subsequently, the
physician may continue decreasing the dose but at a more gradual rate.
In placebo-controlled clinical trials of up to nine weeks'
duration of patients with MDD, a systematic evaluation of
discontinuation symptoms in patients taking duloxetine following abrupt
discontinuation found the following symptoms occurring at a rate greater
than or equal to 2% and at a significantly higher rate in
duloxetine-treated patients compared to those discontinuing from
placebo: dizziness, nausea, headache, paresthesia, vomiting,
irritability, and nightmare.
Suicidality
The FDA requires all antidepressants, including duloxetine, to carry a
black box warning
stating that antidepressants may increase the risk of suicide in
persons younger than 25. This warning is based on statistical analyses
conducted by two independent groups of the FDA experts that found a
2-fold increase of the suicidal ideation and behavior in children and
adolescents, and 1.5-fold increase of suicidality in the 18–24 age
group.
To obtain
statistically significant results the FDA had to combine the results of 295 trials of 11 antidepressants for psychiatric indications. As
suicidal ideation
and behavior in clinical trials are rare, the results for any drug
taken separately usually do not reach statistical significance.
In 2005 the United States FDA released a public health advisory
noting that there had been 11 reports of suicide attempts and 3 reports
of suicidality within the mostly middle-aged women participating in the
open label extension trials of duloxetine for the treatment of stress
urinary incontinence. The FDA described the potential role of
confounding social stressors "unclear". The suicide attempt rate in the
SUI study population (based on 9,400 patients) was calculated to be 400
per 100,000 person years. This rate is greater than the suicide attempt
rate among middle-aged U.S. women that has been reported in published
studies, i.e., 150 to 160 per 100,000 person years. In addition, one
death from suicide was reported in a Cymbalta clinical pharmacology
study in a healthy female volunteer without SUI. No increase in
suicidality was reported in controlled trials of Cymbalta for depression
or diabetic neuropathic pain.
Postmarketing reports
Reported
adverse events that were temporally correlated to duloxetine therapy
include rash, reported rarely, and the following adverse events,
reported very rarely:
alanine aminotransferase increased,
alkaline phosphatase increased,
anaphylactic reaction,
angioneurotic edema,
aspartate aminotransferase increased,
bilirubin increased,
glaucoma,
hepatotoxicity,
hyponatremia,
jaundice,
orthostatic hypotension (especially at the initiation of treatment),
Stevens–Johnson syndrome,
syncope (especially at initiation of treatment), and
urticaria.
Pharmacology
Mechanism of action
Duloxetine inhibits the reuptake of serotonin and norepinephrine (NE)
in the central nervous system. Duloxetine increases dopamine (DA)
specifically in the prefrontal cortex, where there are few DA reuptake
pumps, via the inhibition of NE reuptake pumps (NET), which is believed
to mediate reuptake of DA and NE.
Duloxetine has no significant affinity for dopaminergic, cholinergic,
histaminergic, opioid, glutamate, and GABA reuptake transporters,
however, and can therefore be considered to be a selective reuptake
inhibitor at the 5-HT and NE transporters. Duloxetine undergoes
extensive
metabolism, but the major circulating metabolites do not contribute significantly to the pharmacologic activity.
Major depressive disorder is believed to be due in part to an
increase in pro-inflammatory cytokines within the central nervous
system. Antidepressants including ones with a similar mechanism of
action as duloxetine, i.e. serotonin metabolism inhibition, cause a
decrease in proinflammatory
cytokine
activity and an increase in anti-inflammatory cytokines; this mechanism
may apply to duloxetine in its effect on depression but research on
cytokines specific to duloxetine therapy is lacking.
The analgesic properties of duloxetine in the treatment of
diabetic neuropathy and central pain syndromes such as fibromyalgia are
believed to be due to sodium ion channel blockade.
Pharmacokinetics
Absorption:
Duloxetine is acid labile, and is formulated with enteric coating to
prevent degradation in the stomach. Duloxetine has good oral
bioavailability, averaging 50% after one 60 mg dose. There is an average
2-hour lag until absorption begins with maximum plasma concentrations
occurring about 6 hours post dose. Food does not affect the Cmax of duloxetine, but delays the time to reach peak concentration from 6 to 10 hours.
Distribution: Duloxetine is highly bound (>90%) to
proteins in human plasma, binding primarily to albumin and α1-acid
glycoprotein. Volume of distribution is 1640L.
Metabolism: Duloxetine undergoes predominately hepatic
metabolism via two cytochrome P450 isozymes, CYP2D6 and CYP1A2.
Circulating metabolites are pharmacologically inactive.
Elimination: Duloxetine has an
elimination half-life of about 12 hours (range 8 to 17 hours) and its pharmacokinetics are dose proportional over the
therapeutic range.
Steady-state is usually achieved after 3 days. Only trace amounts
(<1 20="" 70="" about="" and="" appears="" approx.="" are="" as="" dose="" duloxetine="" excreted="" feces.="" in="" metabolites="" most="" of="" p="" present="" the="" unchanged="" urine="" with="">
History
Cymbalta (duloxetine) 60mg
Duloxetine was created by Lilly researchers. David Robertson; David Wong, a co-discoverer of
fluoxetine; and Joseph Krushinski are listed as inventors on the
patent application filed in 1986 and granted in 1990. The first publication on the discovery of the
racemic form of duloxetine known as LY227942, was made in 1988. The (+)-
enantiomer
of LY227942, assigned LY248686, was chosen for further studies, because
it inhibited serotonin reuptake in rat synaptosomes to twice the degree
of the (–)-enantiomer. This molecule was subsequently named duloxetine.
In 2001, Lilly filed a
New Drug Application (NDA) for duloxetine with the US
Food and Drug Administration.
In 2003, however, the FDA "recommended this application as not
approvable from the manufacturing and control standpoint" because of
"significant cGMP (current
Good Manufacturing Practice)
violations at the finished product manufacturing facility" of Eli Lilly
in Indianapolis. Additionally, "potential liver toxicity" and
QTc interval
prolongation appeared as a concern. The FDA experts concluded that
"duloxetine can cause hepatotoxicity in the form of transaminase
elevations. It may also be a factor in causing more severe liver injury,
but there are no cases in the NDA database that clearly demonstrate
this. Use of duloxetine in the presence of ethanol may potentiate the
deleterious effect of ethanol on the liver." The FDA also recommended
"routine
blood pressure
monitoring" at the new highest recommended dose of 120 mg, "where 24%
patients had one or more blood pressure readings of 140/90 vs. 9% of
placebo patients."
After the manufacturing issues were resolved, the
liver toxicity
warning included in the prescribing information, and the follow-up
studies showed that duloxetine does not cause QTc interval prolongation,
duloxetine was approved by the FDA for depression and diabetic
neuropathy in 2004. In 2007,
Health Canada approved duloxetine for the treatment of depression and diabetic peripheral neuropathic pain.
Duloxetine was approved for use of stress urinary incontinence
(SUI) in the EU in 2004. In 2005, Lilly withdrew the duloxetine
application for stress urinary incontinence (SUI) in the U.S., stating
that discussions with the FDA indicated "the agency is not prepared at
this time to grant approval ... based on the data package submitted." A
year later Lilly abandoned the pursuit of this indication in the U.S.
market.
The FDA approved duloxetine for the treatment of generalized anxiety disorder in February 2007.
Cymbalta generated sales of nearly $5 billion in 2012 with $4
billion of that in the U.S., but its patent protection terminated
January 1, 2014. Lilly received a six-month extension beyond June 30,
2013 after testing for the treatment of depression in adolescents, which
may produce $1.5 billion in added sales. It was the most prescribed antidepressant in 2013–14.
The first generic duloxetine was marketed by
Dr. Reddy.