From Wikipedia, the free encyclopedia

The weather station outside of the Atomic Testing Museum on a hot summer day. Displayed background gamma radiation level is 9.8 μR/h (0.82 mSv/a) This is very close to the world average background radiation of 0.87 mSv/a from cosmic and terrestrial sources.
Background radiation is the ubiquitous ionizing radiation that people on the planet Earth are exposed to, including natural and artificial sources.
Both natural and artificial background radiation varies depending on location and altitude.
| Radiation source | World[1] | USA[2] | Japan[3] | Remark |
|---|---|---|---|---|
| Inhalation of air | 1.26 | 2.28 | 0.40 | mainly from radon, depends on indoor accumulation |
| Ingestion of food & water | 0.29 | 0.28 | 0.40 | (K-40, C-14, etc.) |
| Terrestrial radiation from ground | 0.48 | 0.21 | 0.40 | depends on soil and building material |
| Cosmic radiation from space | 0.39 | 0.33 | 0.30 | depends on altitude |
| sub total (natural) | 2.40 | 3.10 | 1.50 | sizeable population groups receive 10-20 mSv |
| Medical | 0.60 | 3.00 | 2.30 | world-wide figure excludes radiotherapy; US figure is mostly CT scans and nuclear medicine. |
| Consumer items | - | 0.13 | cigarettes, air travel, building materials, etc. | |
| Atmospheric nuclear testing | 0.005 | - | 0.01 | peak of 0.11 mSv in 1963 and declining since; higher near sites |
| Occupational exposure | 0.005 | 0.005 | 0.01 | world-wide average to all workers is 0.7 mSv, mostly due to radon in mines;[1] US is mostly due to medical and aviation workers.[2] |
| Chernobyl accident | 0.002 | - | 0.01 | peak of 0.04 mSv in 1986 and declining since; higher near site |
| Nuclear fuel cycle | 0.0002 | 0.001 | up to 0.02 mSv near sites; excludes occupational exposure | |
| Other | - | 0.003 | Industrial, security, medical, educational, and research | |
| sub total (artificial) | 0.61 | 3.14 | 2.33 | |
| Total | 3.01 | 6.24 | 3.83 | millisievert per year |
Natural background radiation
Radioactive material is found throughout nature. Detectable amounts occur naturally in soil, rocks, water, air, and vegetation, from which it is inhaled and ingested into the body. In addition to this internal exposure, humans also receive external exposure from radioactive materials that remain outside the body and from cosmic radiation from space. The worldwide average natural dose to humans is about 2.4 millisievert (mSv) per year.[1] This is four times more than the worldwide average artificial radiation exposure, which in the year 2008 amounted to about 0.6 mSv per year. In some rich countries like the US and Japan, artificial exposure is, on average, greater than the natural exposure, due to greater access to medical imaging. In Europe, average natural background exposure by country ranges from under 2 mSv annually in the United Kingdom to more than 7 mSv annually for some groups of people in Finland.[4]Air
The biggest source of natural background radiation is airborne radon, a radioactive gas that emanates from the ground. Radon and its isotopes, parent radionuclides, and decay products all contribute to an average inhaled dose of 1.26 mSv/a. Radon is unevenly distributed and varies with weather, such that much higher doses apply to many areas of the world, where it represents a significant health hazard. Concentrations over 500 times higher than the world average have been found inside buildings in Scandinavia, the United States, Iran, and the Czech Republic.[5] Radon is a decay product of uranium, which is relatively common in the Earth's crust, but more concentrated in ore-bearing rocks scattered around the world. Radon seeps out of these ores into the atmosphere or into ground water or infiltrates into buildings. It can be inhaled into the lungs, along with its decay products, where they will reside for a period of time after exposure.Although radon is naturally occurring, exposure can be enhanced or diminished by human activity, notably house construction. A poorly sealed basement in an otherwise well insulated house can result in the accumulation of radon within the dwelling, exposing its residents to high concentrations. The widespread construction of well insulated and sealed homes in the northern industrialized world has led to radon becoming the primary source of background radiation in some localities in northern North America and Europe.[citation needed] Since it is heavier than air, radon tends to collect in basements and mines. Basement sealing and suction ventilation reduce exposure. Some building materials, for example lightweight concrete with alum shale, phosphogypsum and Italian tuff, may emanate radon if they contain radium and are porous to gas.[5]
Radiation exposure from radon is indirect. Radon has a short half-life (4 days) and decays into other solid particulate radium-series radioactive nuclides. These radioactive particles are inhaled and remain lodged in the lungs, causing continued exposure. Radon is thus the second leading cause of lung cancer after smoking, and accounts for 15,000 to 22,000 cancer deaths per year in the US alone.[6][better source needed]
About 100,000 Bq/m3 of radon was found in Stanley Watras's basement in 1984.[7][8] He and his neighbours in Boyertown, Pennsylvania, United States may hold the record for the most radioactive dwellings in the world. International radiation protection organizations estimate that a committed dose may be calculated by multiplying the equilibrium equivalent concentration (EEC) of radon by a factor of 8 to 9 nSv·m3/Bq·h and the EEC of thoron by a factor of 40 nSv·m3/Bq·h.[1]
Most of the atmospheric background is caused by radon and its decay products. The gamma spectrum shows prominent peaks at 609, 1120, and 1764 keV, belonging to bismuth-214, a radon decay product. The atmospheric background varies greatly with wind direction and meteorological conditions. Radon also can be released from the ground in bursts and then form "radon clouds" capable of traveling tens of kilometers.[9]
Cosmic radiation

The Earth and all living things on it are constantly bombarded by radiation from outer space. This radiation primarily consists of positively charged ions from protons to iron and larger nuclei derived sources outside our solar system. This radiation interacts with atoms in the atmosphere to create an air shower of secondary radiation, including X-rays, muons, protons, alpha particles, pions, electrons, and neutrons. The immediate dose from cosmic radiation is largely from muons, neutrons, and electrons, and this dose varies in different parts of the world based largely on the geomagnetic field and altitude. This radiation is much more intense in the upper troposphere, around 10 km altitude, and is thus of particular concern for airline crews and frequent passengers, who spend many hours per year in this environment. During their flights airline crews typically get an extra dose on the order of 2.2 mSv (220 mrem) per year.[10]
Similarly, cosmic rays cause higher background exposure in astronauts than in humans on the surface of Earth. Astronauts in low orbits, such as in the International Space Station or the Space Shuttle, are partially shielded by the magnetic field of the Earth, but also suffer from the Van Allen radiation belt which accumulates cosmic rays and results from the Earth's magnetic field. Outside low Earth orbit, as experienced by the Apollo astronauts who traveled to the Moon, this background radiation is much more intense, and represents a considerable obstacle to potential future long term human exploration of the moon or Mars.
Cosmic rays also cause elemental transmutation in the atmosphere, in which secondary radiation generated by the cosmic rays combines with atomic nuclei in the atmosphere to generate different nuclides. Many so-called cosmogenic nuclides can be produced, but probably the most notable is carbon-14, which is produced by interactions with nitrogen atoms. These cosmogenic nuclides eventually reach the Earth's surface and can be incorporated into living organisms. The production of these nuclides varies slightly with short-term variations in solar cosmic ray flux, but is considered practically constant over long scales of thousands to millions of years. The constant production, incorporation into organisms and relatively short half-life of carbon-14 are the principles used in radiocarbon dating of ancient biological materials such as wooden artifacts or human remains.
The cosmic radiation at sea level usually manifests as 511 keV gamma rays from annihilation of positrons created by nuclear reactions of high energy particles and gamma rays. The intensity of cosmic ray background increases rapidly with altitude, and at few kilometers above sea the cosmic rays dominate the spectrum and drown the other natural sources. At higher altitudes there is also the contribution of continuous bremsstrahlung spectrum.[9]
Terrestrial sources
Terrestrial radiation, for the purpose of the table above, only includes sources that remain external to the body. The major radionuclides of concern are potassium, uranium and thorium and their decay products, some of which, like radium and radon are intensely radioactive but occur in low concentrations. Most of these sources have been decreasing, due to radioactive decay since the formation of the Earth, because there is no significant amount currently transported to the Earth.Thus, the present activity on earth from uranium-238 is only half as much as it originally was because of its 4.5 billion year half-life, and potassium-40 (half-life 1.25 billion years) is only at about 8% of original activity. The effects on humans of the actual diminishment (due to decay) of these isotopes is minimal however. This is because humans evolved too recently for the difference in activity over a fraction of a half-life to be significant. Put another way, human history is so short in comparison to a half-life of a billion years, that the activity of these long-lived isotopes has been effectively constant throughout our time on this planet.
In addition, many shorter half-life and thus more intensely radioactive isotopes have not decayed out of the terrestrial environment, however, because of natural on-going production of them. Examples of these are radium-226 (decay product of uranium-238) and radon-222 (a decay product of radium-226).
Thorium and uranium primarily undergo alpha and beta decay, and aren't easily detectable. However, many of their daughter products are strong gamma emitters. Thorium-232 is detectable via a 239 keV peak from lead-212, 511, 583 and 2614 keV from thallium-208, and 911 and 969 keV from actinium-228. Uranium-233 is similar but lacks the actinium-228 peak, which distinguishes it from thorium-232. Uranium-238 manifests as 609, 1120, and 1764 keV peaks of bismuth-214 (cf. the same peak for atmospheric radon). Potassium-40 is detectable directly via its 1461 keV gamma peak.[9]
Above sea and bodies of water the terrestrial background tends to be about 10 times lower. At coastal areas and over fresh water additional contribution is possible from dispersed sediment.[9]
Food and water
Some of the essential elements that make up the human body, mainly potassium and carbon, have radioactive isotopes that add significantly to our background radiation dose. An average human contains about 30 milligrams of potassium-40 (40K) and about 10 nanograms (10−8 g) of carbon-14 (14C),[citation needed] which has a decay half-life of 5,730 years. Excluding internal contamination by external radioactive material, the largest component of internal radiation exposure from biologically functional components of the human body is from potassium-40. The decay of about 4,000 nuclei of 40K per second[11] makes potassium the largest source of radiation in terms of number of decaying atoms. The energy of beta particles produced by 40K is also about 10 times more powerful than the beta particles from 14C decay. 14C is present in the human body at a level of 3700 Bq with a biological half-life of 40 days.[12] There are about 1,200 beta particles per second produced by the decay of 14C. However, a 14C atom is in the genetic information of about half the cells, while potassium is not a component of DNA. The decay of a 14C atom inside DNA in one person happens about 50 times per second, changing a carbon atom to one of nitrogen.[13] The global average internal dose from radionuclides other than radon and its decay products is 0.29 mSv/a, of which 0.17 mSv/a comes from 40K, 0.12 mSv/a comes from the uranium and thorium series, and 12 μSv/a comes from 14C.[1]Areas with high NBR
Some areas have greater dosage than the country-wide averages.[14] In the world in general, exceptionally high natural background locales include Ramsar in Iran, Guarapari in Brazil, Karunagappalli in India,[15] Arkaroola, South Australia [16] and Yangjiang in China.[17]The highest level of purely natural radiation ever recorded on the Earth's surface was 90 µGy/h on a Brazilian black beach (areia preta in Portuguese) composed of monazite.[18] This rate would convert to 0.8 Gy/a for year-round continuous exposure, but in fact the levels vary seasonally and are much lower in the nearest residences. The record measurement has not been duplicated and is omitted from UNSCEAR's latest reports. Nearby tourist beaches in Guarapari and Cumuruxatiba were later evaluated at 14 and 15 µGy/h.[19][20]
The highest background radiation in an inhabited area is found in Ramsar, primarily due to the use of local naturally radioactive limestone as a building material. The 1000 most exposed residents receive an average external effective radiation dose of 6 mSv per year, (0.6 rem/yr,) six times more than the ICRP recommended limit for exposure to the public from artificial sources.[21] They additionally receive a substantial internal dose from radon. Record radiation levels were found in a house where the effective dose due to ambient radiation fields was 131 mSv/a, (13.1 rem/yr) and the internal committed dose from radon was 72 mSv/a (7.2 rem/yr).[21] This unique case is over 80 times higher than the world average natural human exposure to radiation.
Epidemiological studies are underway to identify health effects associated with the high radiation levels in Ramsar. It is much too early to draw statistically significant conclusions.[21] While so far support for beneficial effects of chronic radiation (like longer lifespan) has not been observed, a protective and adaptive effect is suggested by at least one study whose authors nonetheless caution that data from Ramsar are not yet sufficiently strong to relax existing regulatory dose limits.[22]
Photoelectric
Background radiation doses in the immediate vicinities of particles of high atomic number materials, within the human body, have a small enhancement due to the photoelectric effect.[23]Neutron background
Most of the natural neutron background is a product of cosmic rays interacting with the atmosphere. The neutron energy peaks at around 1 MeV and rapidly drops above. At sea level, the production of neutrons is about 20 neutrons per second per kilogram of material interacting with the cosmic rays (or, about 100-300 neutrons per square meter per second). The flux is dependent on geomagnetic latitude, with a maximum at about 45 degrees. At solar minimums, due to lower solar magnetic field shielding, the flux is about twice as high vs the solar maximum. It also dramatically increases during solar flares. In the vicinity of larger heavier objects, e.g. buildings or ships, the neutron flux measures higher; this is known as "cosmic ray induced neutron signature", or "ship effect" as it was first detected with ships at sea.[9]Artificial background radiation
Medical
The global average human exposure to artificial radiation is 0.6 mSv/a, primarily from medical imaging. This medical component can range much higher, with an average of 3 mSv per year across the USA population.[2] Other human contributors include smoking, air travel, radioactive building materials, historical nuclear weapons testing, nuclear power accidents and nuclear industry operation.A typical chest x-ray delivers 0.02 mSv (2 mrem) of effective dose.[24] A dental x-ray delivers a dose of 5 to 10 µSv[25] The average American receives about 3 mSv of diagnostic medical dose per year; countries with the lowest levels of health care receive almost none. Radiation treatment for various diseases also accounts for some dose, both in individuals and in those around them.
Consumer items
Cigarettes contain polonium-210, originating from the decay products of radon, which stick to tobacco leaves. Heavy smoking results in a radiation dose of 160 mSv/year to localized spots at the bifurcations of segmental bronchi in the lungs from the decay of polonium-210. This dose is not readily comparable to the radiation protection limits, since the latter deal with whole body doses, while the dose from smoking is delivered to a very small portion of the body.[26]Air travel causes increased exposure to cosmic radiation. The average extra dose to flight personnel is 2.19 mSv/year.[27]
Atmospheric nuclear testing
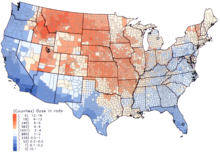
Per capita thyroid doses in the continental United States resulting from all exposure routes from all atmospheric nuclear tests conducted at the Nevada Test Site from 1951-1962.
Frequent above-ground nuclear explosions between the 1940s and 1960s scattered a substantial amount of radioactive contamination. Some of this contamination is local, rendering the immediate surroundings highly radioactive, while some of it is carried longer distances as nuclear fallout; some of this material is dispersed worldwide. The increase in background radiation due to these tests peaked in 1963 at about 0.15 mSv per year worldwide, or about 7% of average background dose from all sources. The Limited Test Ban Treaty of 1963 prohibited above-ground tests, thus by the year 2000 the worldwide dose from these tests has decreased to only 0.005 mSv per year.[28]
Occupational exposure
The ICRP recommends limiting occupational radiation exposure to 50 mSv (5 rem) per year, and 100 mSv (10 rem) in 5 years.[29]At an IAEA conference in 2002, it was recommended that occupational doses below 1–2 mSv per year do not warrant regulatory scrutiny.[30]
Nuclear accidents
Under normal circumstances, nuclear reactors release small amounts of radioactive gases, which cause negligibly-small radiation exposures to the public. Events classified on the International Nuclear Event Scale as incidents typically do not release any additional radioactive substances into the environment. Large releases of radioactivity from nuclear reactors are extremely rare. To the present day, there were two major civilian accidents - the Chernobyl accident and the Fukushima I nuclear accidents - which caused substantial contamination. The Chernobyl accident was the only one to cause immediate deaths.Total doses from the Chernobyl accident ranged from 10 to 50 mSv over 20 years for the inhabitants of the affected areas, with most of the dose received in the first years after the disaster, and over 100 mSv for liquidators. There were 28 deaths from acute radiation syndrome.[31]
Total doses from the Fukushima I accidents were between 1 and 15 mSv for the inhabitants of the affected areas. Thyroid doses for children were below 50 mSv. 167 cleanup workers received doses above 100 mSv, with 6 of them receiving more than 250 mSv (the Japanese exposure limit for emergency response workers).[32]
The average dose from the Three Mile Island accident was 0.01 mSv.[33]
Non-civilian: In addition to the civilian accidents described above, several accidents at early nuclear weapons facilities - such as the Windscale fire, the contamination of the Techa River by the nuclear waste from the Mayak compound, and the Kyshtym disaster at the same compound - released substantial radioactivity into the environment. The Windscale fire resulted in thyroid doses of 5-20 mSv for adults and 10-60 mSv for children.[34] The doses from the accidents at Mayak are unknown.
Nuclear fuel cycle
The Nuclear Regulatory Commission, the United States Environmental Protection Agency, and other U.S. and international agencies, require that licensees limit radiation exposure to individual members of the public to 1 mSv (100 mrem) per year.Other
Coal plants emit radiation in the form of radioactive fly ash which is inhaled and ingested by neighbours, and incorporated into crops. A 1978 paper from Oak Ridge National Laboratory estimated that coal-fired power plants of that time may contribute a whole-body committed dose of 19 µSv/a to their immediate neighbours in a radius of 500 m.[35] The United Nations Scientific Committee on the Effects of Atomic Radiation's 1988 report estimated the committed dose 1 km away to be 20 µSv/a for older plants or 1 µSv/a for newer plants with improved fly ash capture, but was unable to confirm these numbers by test.[36] When coal is burned, uranium, thorium and all the uranium daughters accumulated by disintegration — radium, radon, polonium — are released.[37]Radioactive materials previously buried underground in coal deposits are released as fly ash or, if fly ash is captured, may be incorporated into concrete manufactured with fly ash.
Other usage
In other contexts, background radiation may simply be any radiation that is pervasive, whether ionizing or not. A particular example of this is the cosmic microwave background radiation, a nearly uniform glow that fills the sky in the microwave part of the spectrum; stars, galaxies and other objects of interest in radio astronomy stand out against this background.In a laboratory, background radiation refers to the measured value from any sources that affect an instrument when a radiation source sample is not being measured. This background rate, which must be established as a stable value by multiple measurements, usually before and after sample measurement, is subtracted from the rate measured when the sample is being measured.
Background radiation for occupational doses measured for workers is all radiation dose that is not measured by radiation dose measurement instruments in potential occupational exposure conditions. This includes both "natural background radiation" and any medical radiation doses. This value is not typically measured or known from surveys, such that variations in the total dose to individual workers is not known. This can be a significant confounding factor in assessing radiation exposure effects in a population of workers who may have significantly different natural background and medical radiation doses. This is most significant when the occupational doses are very low.

